filmov
tv
Electrolytic vs Galvanic (Voltaic) Cell | Electrochemistry

Показать описание
This video gives you an in-depth comparison of the Galvanic/Voltaic electrochemical cell and the Electrolytic cell that operate on redox reactions to convert chemical energy into electrical energy and vice versa.
You’ll learn how to determine the oxidation and reduction half-reactions at both the anode and cathode, how to identify the charges on the electrodes in each cell, as well as the importance of the salt bridge in maintaining the neutrality of a half-cell solution.
You’ll also get a detailed review of the components of each type of electrochemical cell, as I show you exactly what’s happening with each species in the reaction, where the electrons are flowing and how the cell is being powered.
↪ Additional Links & Resources ↩
- - - - - - - - - - - - - - - - - - - - - - - -
⏱ In this video:
0:18 Galvanic/Voltaic Cell
0:52 Zn/Cu half reaction
5:09 Salt Bridge Na/K
6:12 Electrolytic cell
6:43 Na/Cl half reaction
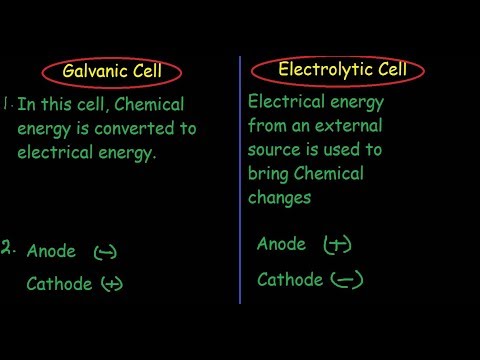
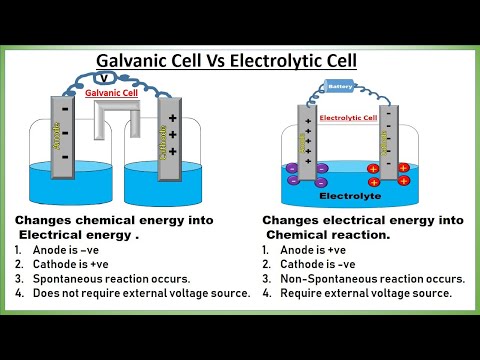
11:44 Galvanic and Electrolytic comparison
- - - - - - - - - - - - - - - - - - - - - - - -
Looking for guidance on how to tailor your MCAT Self-Study journey to fit your unique background, experience, and personal goals without feeling alone in the process?
That’s what my new MCAT program is all about, join me here:
🔔 Subscribe to my channel so you don’t miss out on any new videos 🔔
Комментарии
 0:13:00
0:13:00
 0:04:10
0:04:10
 0:09:04
0:09:04
 0:27:42
0:27:42
 0:06:55
0:06:55
 0:12:59
0:12:59
 0:23:35
0:23:35
 0:17:43
0:17:43
 0:18:27
0:18:27
 0:06:21
0:06:21
 0:06:04
0:06:04
 0:02:33
0:02:33
 0:11:19
0:11:19
 0:02:04
0:02:04
 0:01:01
0:01:01
 0:00:15
0:00:15
 0:01:00
0:01:00
 0:04:56
0:04:56
 0:24:25
0:24:25
 0:06:24
0:06:24
 0:02:26
0:02:26
 0:23:08
0:23:08
 0:00:22
0:00:22
 0:15:32
0:15:32