filmov
tv
Introduction to Galvanic Cells & Voltaic Cells
Показать описание
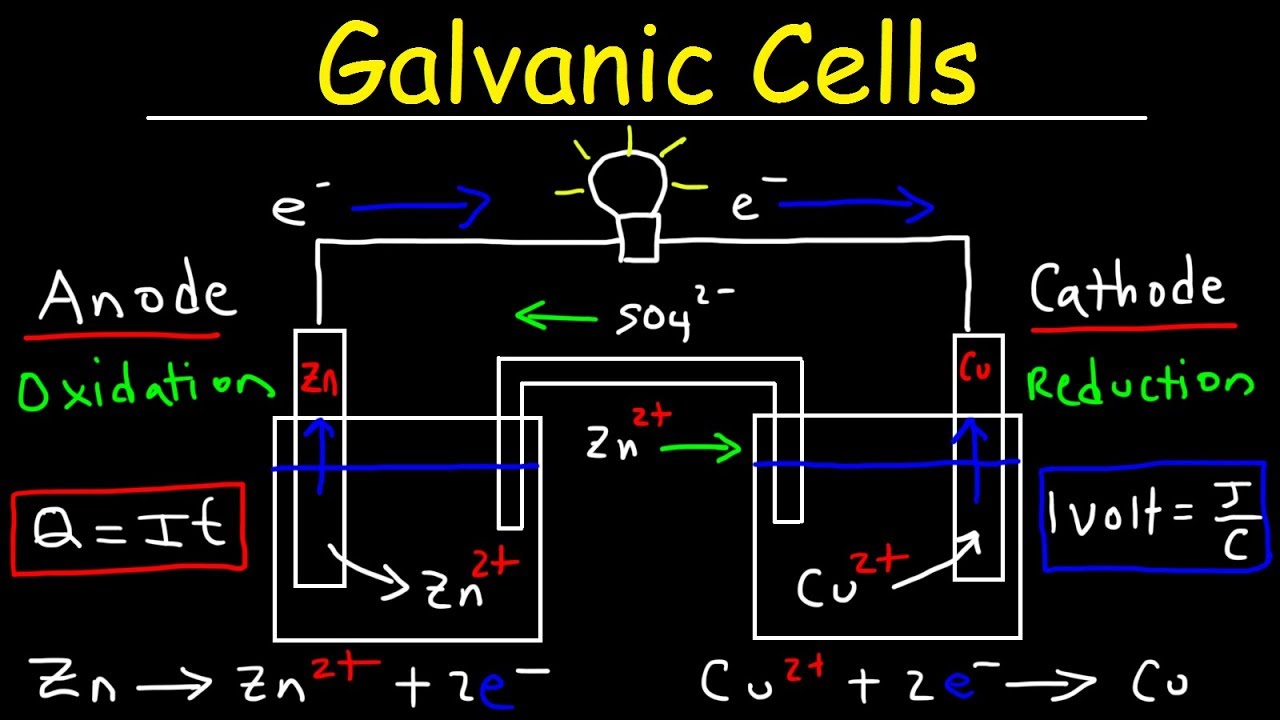
This chemistry video tutorial provides a basic introduction into electrochemical cells such as galvanic cells also known as voltaic cells. A galvanic cell is device such as a battery which converts chemical energy into electrical energy. A galvanic is made using two electrodes known as the anode and the cathode. Oxidation occurs at the anode and reduction occurs in the cathode. The electrons are written on the right of an oxidation half-reaction and they are located on the left side of a reduction half-reaction. Oxidation involves the loss of electrons and reduction is associated with the gain of electrons. Electrons always flow from the anode to the cathode. The purpose of the salt bridge is to prevent the build up of charge. Cations flow through the salt bridge toward the cathode and anions flow toward the anode. This electrochemistry explains completely how a galvanic cell works. It discusses how to write the overall reaction from the half reactions and how to represent the cell using standard line notation or cell notation. The electromotive force of a galvanic cell is measured in volts and 1 volt is 1 joule per coulomb. Voltage represents the work that can be done per coulomb of electric charge. Electric current is the rate at which electric charge is transferred. This video discusses how to increase the voltage and current delivered by a galvanic cell.
Electrochemistry - Free Formula Sheet:
Chemistry 2 Final Exam Review:
Chemistry PDF Worksheets:
_______________________________
Intro to Galvanic & Voltaic Cells:
How To Draw Galvanic Cells:
Standard Reduction Potentials:
Cell Potential Problems:
Cell Notation Problems:
___________________________________
Concentration Cells:
Cell Potential & Gibbs Free Energy:
Cell Potential & Equilibrium K:
Nernst Equation:
Electrolysis of Water:
_____________________________________
Electrolysis of Sodium Chloride:
Electrolysis & Electroplating Problems:
Electrochemistry Practice Problems:
SAT Chemistry Subject Test Review:
Carbon -14 Dating:
Beer Lambert's Law:
______________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Electrochemistry - Free Formula Sheet:
Chemistry 2 Final Exam Review:
Chemistry PDF Worksheets:
_______________________________
Intro to Galvanic & Voltaic Cells:
How To Draw Galvanic Cells:
Standard Reduction Potentials:
Cell Potential Problems:
Cell Notation Problems:
___________________________________
Concentration Cells:
Cell Potential & Gibbs Free Energy:
Cell Potential & Equilibrium K:
Nernst Equation:
Electrolysis of Water:
_____________________________________
Electrolysis of Sodium Chloride:
Electrolysis & Electroplating Problems:
Electrochemistry Practice Problems:
SAT Chemistry Subject Test Review:
Carbon -14 Dating:
Beer Lambert's Law:
______________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:27:42
0:27:42
 0:06:00
0:06:00
 0:03:41
0:03:41
 0:23:35
0:23:35
 0:05:54
0:05:54
 0:14:57
0:14:57
 0:04:10
0:04:10
 0:16:37
0:16:37
 0:27:00
0:27:00
 0:15:09
0:15:09
 0:09:04
0:09:04
 0:56:52
0:56:52
 0:06:21
0:06:21
 0:06:44
0:06:44
 0:00:33
0:00:33
 0:04:48
0:04:48
 0:08:16
0:08:16
 0:26:40
0:26:40
 0:12:52
0:12:52
 0:18:18
0:18:18
 0:00:10
0:00:10
 0:10:56
0:10:56
 0:06:55
0:06:55
 0:06:59
0:06:59