filmov
tv
Galvanic Cells (Voltaic Cells)

Показать описание
All about Galvanic Cells, which are also called Voltaic Cells. These are devices that use a chemical reaction to create electricity. Moving electrons create electricity, and electrons flow from the anode, the site of oxidation, to the cathode, the site of reduction. The galvanic or voltaic cell also includes a salt bridge, which helps to balance charge, and lets ions move between the two half cells. In this video, we'll talk about oxidation, reduction, cathodes, and anodes. We'll write redox half reactions for the two half cells, which show the gain and loss of electrons.
Galvanic Cells (Voltaic Cells)
Introduction to Galvanic Cells & Voltaic Cells
Galvanic cells explained -in UNDER 5 MINUTES.
Voltaic cell | How does it work?
Galvanic Cell.swf
Electrochemistry
Introduction to galvanic/voltaic cells | Chemistry | Khan Academy
Galvanic Cell Or Voltaic Cell | Salt Bridge | Animation | Chemistry Ask
How To Draw Galvanic Cells and Voltaic Cells - Electrochemistry
Galvanic Cells (Voltaic Cells): Part One
Electrochemistry: Crash Course Chemistry #36
Galvanic (voltaic) cells | Applications of thermodynamics | AP Chemistry | Khan Academy
Galvanic / Voltaic Electrochemical Cells
A Voltaic Cell or Galvanic Cell Animation
Galvanic cell or Voltaic cell :Definition ,Construction and Working | Galvanic Cell Animation Video
Introduction to electrolysis | Redox reactions and electrochemistry | Chemistry | Khan Academy
Physically Constructing a Galvanic Cell (Electrochemistry)
Electrochemistry Galvanic/Voltaic Cell Battery Made Super Simple! MCAT Chemistry
Standard Zinc-Copper Voltaic Cell with Salt Bridge
Introduction to Electrochemistry
Super-corroding Galvanic Cell used to Heat Soldier’s Meals!
Galvanic Cells (Voltaic Cells) | Worked Example with Cathode, Anode, and Salt Bridge
Electrolytic vs Galvanic (Voltaic) Cell | Electrochemistry
Construction and working of Galvanic cell | Voltic cell | Galvanic cell | Electrochemistry
Комментарии
 0:23:35
0:23:35
 0:27:42
0:27:42
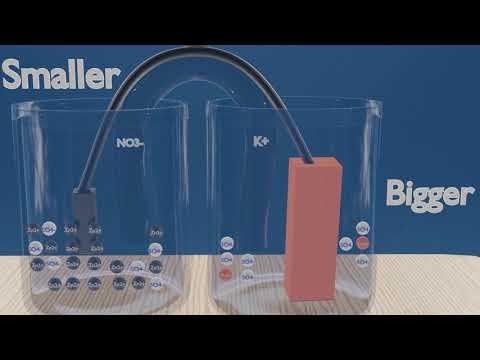 0:03:41
0:03:41
 0:04:10
0:04:10
 0:02:08
0:02:08
 0:06:21
0:06:21
 0:06:00
0:06:00
 0:07:52
0:07:52
 0:15:09
0:15:09
 0:16:29
0:16:29
 0:09:04
0:09:04
 0:09:12
0:09:12
 0:11:19
0:11:19
 0:02:52
0:02:52
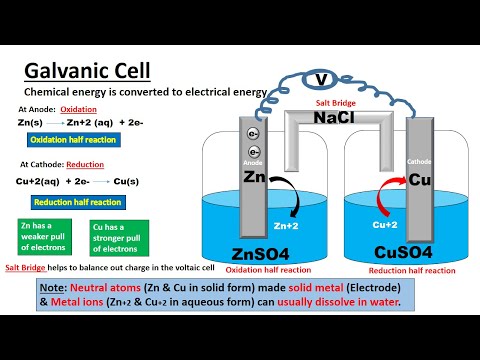 0:09:43
0:09:43
 0:06:55
0:06:55
 0:06:30
0:06:30
 0:19:08
0:19:08
 0:04:52
0:04:52
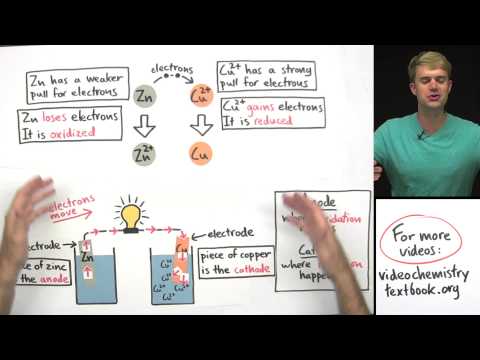 0:16:37
0:16:37
 0:00:33
0:00:33
 0:14:05
0:14:05
 0:13:00
0:13:00
 0:25:42
0:25:42