filmov
tv
Limiting Reagents and Percent Yield

Показать описание
Chemistry doesn't always work perfectly, silly. Molecules are left over when one thing runs out! Also we never get all of the products that we thought we might by doing the math. You gotta know about the limiting reagents and the percent yield! Don't worry, it's as easy as bologna sandwiches.
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Limiting Reagents and Percent Yield
Practice Problem: Limiting Reagent and Percent Yield
How to Find Theoretical Yield (2023)
Limiting Reactants - The FAST Way!!
GCSE Chemistry - What is a Limiting Reactant? Limiting/Excess Reactants Explained #27
Limiting Reactant Practice Problems
Introduction to Limiting Reactant and Excess Reactant
How To Calculate Theoretical Yield and Percent Yield
Limiting Reactant and Percent Yield - Example Problem
Limiting Reagents and Percent Yield
Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
Limiting Reagents and Percent Yields
How to Find Limiting Reactants | How to Pass Chemistry
Limiting Reagent and Percent Yield
Limiting Reactant Grade 11: Practice
Percent Yield Made Easy: Stoichiometry Tutorial Part 4
Limiting Reactant and Percent Yield Lab
Limiting Reagent, Theoretical Yield, and Percent Yield
Limiting Reagents and Percent Yield
Limiting Reactants & Percent Yield
Limiting Reagent Made Easy: Stoichiometry Tutorial Part 5
Limiting and Excess Reagent, Actual, Theoritical and Percentage Yield of Products. #chemistry
How To Calculate Theoretical Yield and Percent Yield
Limiting Reactant and Percent Yield Lab
Комментарии
 0:04:35
0:04:35
 0:09:08
0:09:08
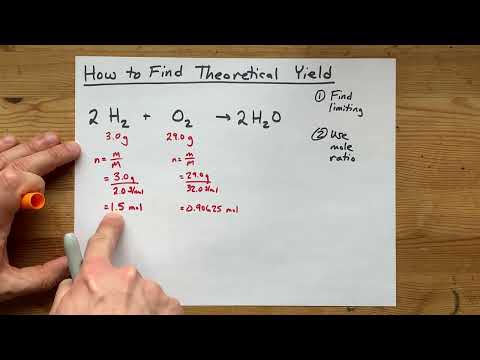 0:05:22
0:05:22
 0:01:00
0:01:00
 0:04:16
0:04:16
 0:18:52
0:18:52
 0:16:58
0:16:58
 0:06:24
0:06:24
 0:12:07
0:12:07
 0:01:59
0:01:59
 0:34:54
0:34:54
 0:09:34
0:09:34
 0:08:52
0:08:52
 0:12:55
0:12:55
 0:14:05
0:14:05
 0:07:45
0:07:45
 0:00:23
0:00:23
 0:10:43
0:10:43
 0:04:01
0:04:01
 0:15:32
0:15:32
 0:08:10
0:08:10
 0:30:29
0:30:29
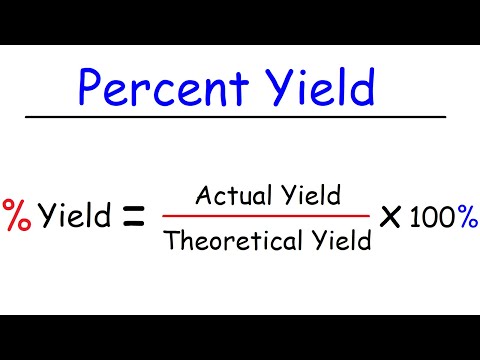 0:11:03
0:11:03
 0:02:05
0:02:05