filmov
tv
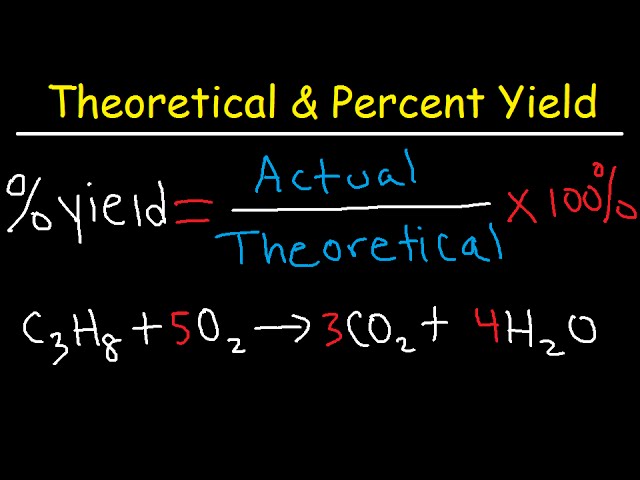
How To Calculate Theoretical Yield and Percent Yield
Показать описание
This video shows you how to calculate the theoretical and percent yield in chemistry. The theoretical yield is the maximum amount of product that can be produced in a reaction. The percent yield is equal to the actual yield divided by the theoretical yield times 100%.
Full-Length Math & Science Videos:
Stoichiometry Formula Sheet:
___________________________________
Introduction to Moles:
How To Calculate The Molar Mass:
How To Convert Grams to Moles:
How To Convert Moles to Grams:
Moles to Atoms Conversion:
Grams to Molecules Conversion:
_________________________________
Grams to Atoms:
Moles, Atoms, & Grams Conversions:
How To Balance Chemical Equations:
Stoichiometry - Basic Introduction:
Avogadro's Number:
_________________________________
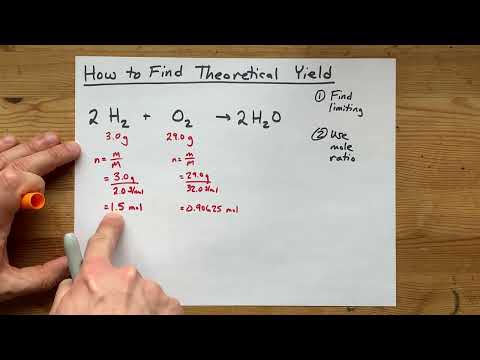
Limiting Reactant Problems:
Excess Reactant Problems:
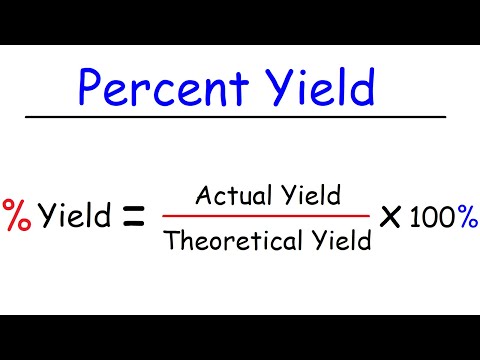
Theoretical & Percent Yield:
Percent Yield - More Examples:
Percent Error:
_________________________________
Percent Composition By Mass:
Empirical Formula Problems:
Empirical Formula - Hydrated Compounds:
Combustion Analysis:
Stoichiometry Practice Test:
_______________________________________
Final Exams and Video Playlists:
Full-Length Math & Science Videos:
Stoichiometry Formula Sheet:
___________________________________
Introduction to Moles:
How To Calculate The Molar Mass:
How To Convert Grams to Moles:
How To Convert Moles to Grams:
Moles to Atoms Conversion:
Grams to Molecules Conversion:
_________________________________
Grams to Atoms:
Moles, Atoms, & Grams Conversions:
How To Balance Chemical Equations:
Stoichiometry - Basic Introduction:
Avogadro's Number:
_________________________________
Limiting Reactant Problems:
Excess Reactant Problems:
Theoretical & Percent Yield:
Percent Yield - More Examples:
Percent Error:
_________________________________
Percent Composition By Mass:
Empirical Formula Problems:
Empirical Formula - Hydrated Compounds:
Combustion Analysis:
Stoichiometry Practice Test:
_______________________________________
Final Exams and Video Playlists:
Комментарии
 0:06:24
0:06:24
 0:05:22
0:05:22
 0:11:03
0:11:03
 0:04:15
0:04:15
 0:17:02
0:17:02
 0:10:40
0:10:40
 0:21:30
0:21:30
 0:23:46
0:23:46
 0:04:35
0:04:35
 0:04:54
0:04:54
 0:07:45
0:07:45
 0:05:29
0:05:29
 0:04:10
0:04:10
 0:06:17
0:06:17
 0:12:45
0:12:45
 0:08:25
0:08:25
 0:14:02
0:14:02
 0:10:43
0:10:43
 0:11:52
0:11:52
 0:02:13
0:02:13
 0:07:22
0:07:22
 0:07:15
0:07:15
 0:07:32
0:07:32
 0:05:52
0:05:52