filmov
tv
Percent Yield Made Easy: Stoichiometry Tutorial Part 4
Показать описание
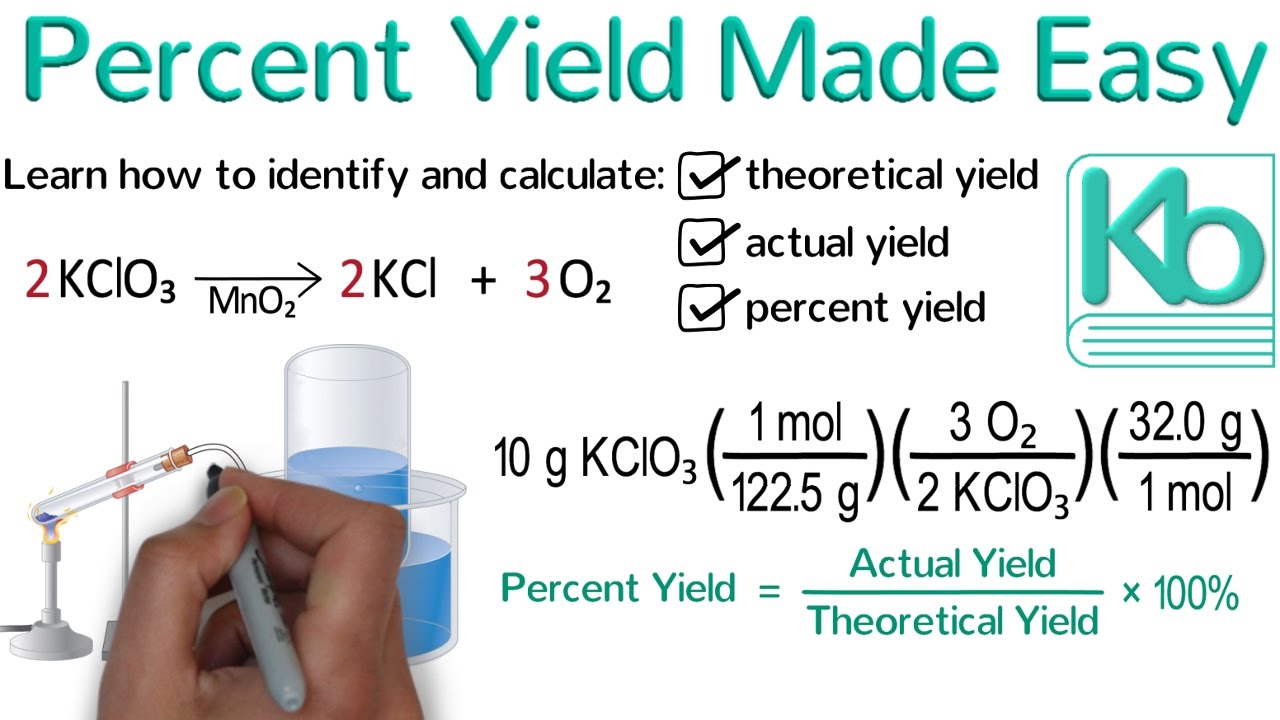
This is a whiteboard animation tutorial that demonstrates how to identify the actual yield of a chemical reaction and how to calculate the theoretical yield and percent yield of a chemical reaction.
Please consider supporting me on Patreon:
The actual yield is the amount of product that is actually produced and isolated from a chemical reaction.
The theoretical yield is the amount of product that could be produced if the reaction worked perfectly. In order to determine the theoretical yield, you will need to perform a stoichiometry calculation (typically grams to grams).
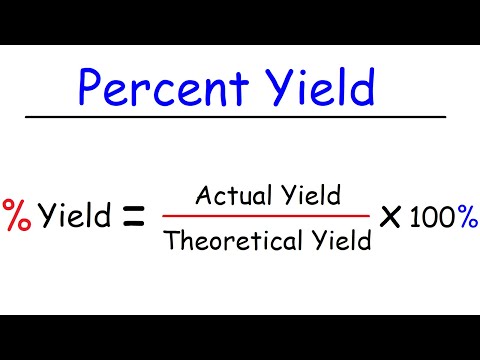
The percent yield tells you how well the reaction worked. It is the actual yield divided by the theoretical yield times 100%.
This is the fourth tutorial in my stoichiometry series.
My goal is to make chemistry easier ;)
#chemistry #madeeasy #stoichiometry #ketzbook #tutorial
Please consider supporting me on Patreon:
The actual yield is the amount of product that is actually produced and isolated from a chemical reaction.
The theoretical yield is the amount of product that could be produced if the reaction worked perfectly. In order to determine the theoretical yield, you will need to perform a stoichiometry calculation (typically grams to grams).
The percent yield tells you how well the reaction worked. It is the actual yield divided by the theoretical yield times 100%.
This is the fourth tutorial in my stoichiometry series.
My goal is to make chemistry easier ;)
#chemistry #madeeasy #stoichiometry #ketzbook #tutorial
Percent Yield Made Easy: Stoichiometry Tutorial Part 4
How To Calculate Theoretical Yield and Percent Yield
Limiting Reagents and Percent Yield
How to Find Theoretical Yield (2023)
GCSE Chemistry - Percentage Yield #33
Stoichiometry Made Easy: Stoichiometry Tutorial Part 1
Chemistry Made Easy: Percent Yield
How To Calculate Theoretical Yield and Percent Yield
Limiting Reactants - The FAST Way!!
STOICHIOMETRY PERCENT REACTION YIELD | Animation
Percent Yield -- EASY!
How to Calculate Percent Yield and Theoretical Yield The Best Way - TUTOR HOTLINE
Chemistry 323: Percent Yield Stoichiometry Problem Solving
percentage yield#chemistry#class 11
How To Calculate The Percent Yield and Theoretical Yield
Stoichiometry: Percent Yield
GCSE CHEMISTRY : PERCENTAGE YIELD
STOICHIOMETRY - Percent Yield Stoichiometry Problems - CLEAR & EASY
Theoretical Yield & Percent Yield in Chemistry Explained
Limiting Reactants made EASY | Chemistry tutorial, Percent Yield
Percentage Yield
Limiting Reagent Made Easy: Stoichiometry Tutorial Part 5
Percent Yield Tutorial: Explained + Practice Problems | Crash Chemistry Academy
Stoichiometry in chemistry example problem
Комментарии
 0:07:45
0:07:45
 0:06:24
0:06:24
 0:04:35
0:04:35
 0:05:22
0:05:22
 0:04:54
0:04:54
 0:06:55
0:06:55
 0:05:04
0:05:04
 0:11:03
0:11:03
 0:01:00
0:01:00
 0:03:51
0:03:51
 0:03:54
0:03:54
 0:21:30
0:21:30
 0:40:21
0:40:21
 0:00:08
0:00:08
 0:17:02
0:17:02
 0:06:11
0:06:11
 0:00:57
0:00:57
 0:08:37
0:08:37
 0:23:46
0:23:46
 0:07:42
0:07:42
 0:03:54
0:03:54
 0:08:10
0:08:10
 0:05:54
0:05:54
 0:00:58
0:00:58