filmov
tv
Solving Schrodinger equation for the hydrogen atom

Показать описание
Here, I solve Schrodinger equation for an electron assuming the Coulomb interaction potential between the electron and an atomic nucleus. This leads to quantized energies in the same form as predicted by Bohr's model, but also provides information on the atomic orbitals and the form of electronic wave functions. The degeneracy observed for states with different angular momentum quantum numbers l = 0, 1 ... n-1 for a given n is also discussed.
SOLVING the SCHRODINGER EQUATION | Quantum Physics by Parth G
The Schrödinger Equation Explained in 60 Seconds
Particle in a Box Part 1: Solving the Schrödinger Equation
Schrodinger Equation. Get the Deepest Understanding.
How to Solve Schrodinger Equation Practice Problems
The Schrodinger Equation is (Almost) Impossible to Solve.
The Quantum Harmonic Oscillator Part 2: Solving the Schrödinger Equation
Quantum Mechanics and the Schrödinger Equation
Pilot Dalga Teorisi: Kuantum Teorisinin Deterministik Yorumu
Schrödinger equation for hydrogen
Solving the Schrodinger Equation | Time-Independent Schrodinger Equation
Solving 1D Schrödinger Equation [Part 1] Method of Separation of Variables
How to Solve Schrödinger's Equation using Separation of Variables
The True Meaning of Schrödinger's Equation
Schrodinger Equation Explained - Physics FOR BEGINNERS (can YOU understand this?)
Your Daily Equation #12: The Schrödinger Equation--the Core of Quantum Mechanics
Solving the Schrodinger Equation | Infinite Square Well
Solving the Schrodinger Equation | The Free Particle
The Hydrogen Atom, Part 1 of 3: Intro to Quantum Physics
4. Wave-Particle Duality of Matter; Schrödinger Equation
The Hydrogen Atom, Part 2 of 3: Solving the Schrodinger Equation
What is the Schrödinger Equation? A basic introduction to Quantum Mechanics
Unpacking the Schrödinger Equation
Schrodinger equation solutions to the hydrogen atom
Комментарии
 0:13:04
0:13:04
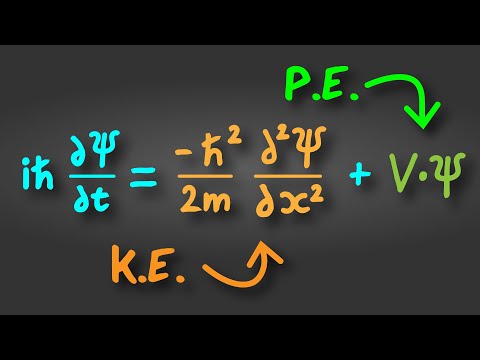 0:01:00
0:01:00
 0:16:35
0:16:35
 0:49:30
0:49:30
 0:26:32
0:26:32
 0:14:08
0:14:08
 0:23:59
0:23:59
 0:06:28
0:06:28
 0:18:57
0:18:57
 0:20:59
0:20:59
 0:03:45
0:03:45
 0:10:19
0:10:19
 0:05:35
0:05:35
 0:12:19
0:12:19
 0:08:45
0:08:45
 0:29:55
0:29:55
 0:04:59
0:04:59
 0:04:30
0:04:30
 0:18:35
0:18:35
 0:46:03
0:46:03
 0:46:00
0:46:00
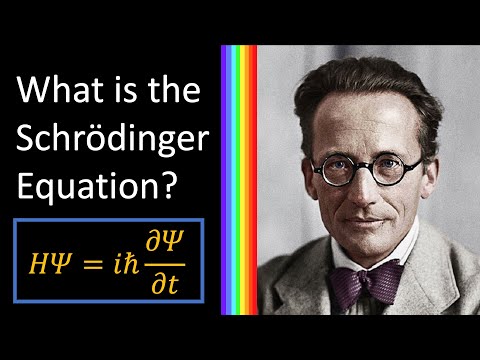 1:27:34
1:27:34
 0:14:13
0:14:13
 0:17:27
0:17:27