filmov
tv
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configuration - Chemistry

Показать описание
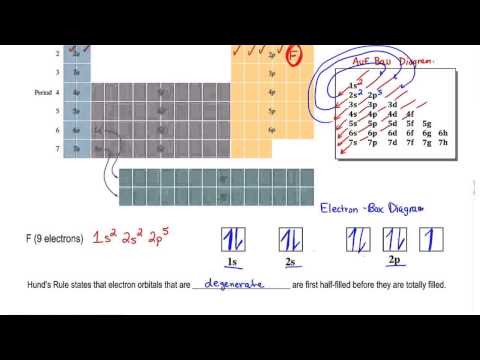
This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion principle and how it relates to orbital diagrams, electron configuration, and quantum numbers. The basic idea of aufbau's principle is that you should fill orbitals with arrows starting from the lowest energy level first. Hund's rule states that you should electrons to degenerate orbitals one at a time with parallel spins. Pauli's exclusion principle states that no two electrons can have the same set of 4 quantum numbers.
Chemistry 1 Final Exam Review:
_______________________________
Electron Configuration - Basic Intro:
Noble Gas Notation:
Electron Configuration Exceptions:
Electron Configuration of Ions:
How To Identify The Element:
Intro to Quantum Numbers:
__________________________________
Orbitals & Atomic Energy Levels:
Maximum Number of Electrons:
Paramagnetic & Diamagnetic Elements:
Aufbau's Principle & Hund's Rule:
Valence Electrons & Periodic Table:
_____________________________________
Effective Nuclear Charge:
Quantum Numbers - Mega Review:
Quantum Numbers - Practice Test:
Lewis Structures Super Review:
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Chemistry 1 Final Exam Review:
_______________________________
Electron Configuration - Basic Intro:
Noble Gas Notation:
Electron Configuration Exceptions:
Electron Configuration of Ions:
How To Identify The Element:
Intro to Quantum Numbers:
__________________________________
Orbitals & Atomic Energy Levels:
Maximum Number of Electrons:
Paramagnetic & Diamagnetic Elements:
Aufbau's Principle & Hund's Rule:
Valence Electrons & Periodic Table:
_____________________________________
Effective Nuclear Charge:
Quantum Numbers - Mega Review:
Quantum Numbers - Practice Test:
Lewis Structures Super Review:
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Комментарии
 0:05:24
0:05:24
 0:10:47
0:10:47
 0:03:54
0:03:54
 0:08:07
0:08:07
 0:04:27
0:04:27
 0:12:13
0:12:13
 0:06:54
0:06:54
 0:00:08
0:00:08
 0:29:52
0:29:52
 0:09:41
0:09:41
 0:01:37
0:01:37
 0:00:33
0:00:33
 0:03:14
0:03:14
 0:03:36
0:03:36
 0:13:17
0:13:17
 0:00:11
0:00:11
 0:00:15
0:00:15
 0:11:26
0:11:26
 0:13:03
0:13:03
 0:10:54
0:10:54
 0:09:56
0:09:56
 0:03:59
0:03:59
 0:02:39
0:02:39
 0:00:43
0:00:43