filmov
tv
Electron Configuration for the First Twenty Elements - Made Simple

Показать описание
A detailed explanation of how to use the Periodic Table to write the electron configurations for the first twenty elements on the Periodic Table (Hydrogen through Calcium).
Using the pattern of orbital blocks, we'll write the electron configurations. Before we can write the electron configs, we first need to know the number of electrons for the each atom. When we write the configuration we'll put these electrons in orbitals around the nucleus of the atom.
Helpful videos:
Electron configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Using the Periodic Table we can quickly write the electron configurations for the first 20 elements (H through Ca).
Using the pattern of orbital blocks, we'll write the electron configurations. Before we can write the electron configs, we first need to know the number of electrons for the each atom. When we write the configuration we'll put these electrons in orbitals around the nucleus of the atom.
Helpful videos:
Electron configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Using the Periodic Table we can quickly write the electron configurations for the first 20 elements (H through Ca).
Electron Configuration - Basic introduction
How to Write the Electron Configuration for an Element in Each Block
Electron Configuration of First 20 Elements | Properties of Matter | Chemistry | FuseSchool
Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchool
Electron Configuration for the First Twenty Elements - Made Simple
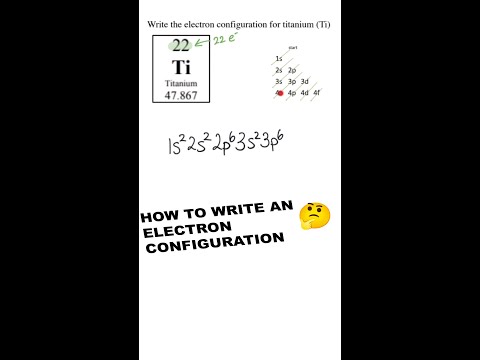
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
Electron configurations for the first period | Chemistry | Khan Academy
Quantum Numbers, Atomic Orbitals, and Electron Configurations
B2 Molecule/MOT
Introduction to Electron Configurations
GCSE Chemistry - Electron Arrangement #8
Electron Configuration
Writing Electron Configurations Using Only the Periodic Table
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configur...
Electron Configurations of Elements
11.4a Writing the electron configuration of a first transition series atom
Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
How to draw Electron-in-box diagrams Electronic Configurations? [GCE A Level Chemistry]
How to Write an Electron Configuration #chemistry #homework #science #shorts #youtubeshorts
SPDF Electronic Configuration Trick | Super trick
Electron Configuration
How to draw electron configurations for the first 20 elements
Electron Configuration - Electron Subshells - Suborbitals - s, p, d, f - Orbitals - Chemistry
9 Science - Electron Configuration
Комментарии
 0:10:19
0:10:19
 0:07:23
0:07:23
 0:03:58
0:03:58
 0:04:59
0:04:59
 0:05:26
0:05:26
 0:01:00
0:01:00
 0:06:22
0:06:22
 0:08:42
0:08:42
 0:15:35
0:15:35
 0:01:46
0:01:46
 0:06:24
0:06:24
 0:10:17
0:10:17
 0:04:52
0:04:52
 0:05:24
0:05:24
 0:03:53
0:03:53
 0:02:05
0:02:05
 0:12:12
0:12:12
 0:04:28
0:04:28
 0:01:00
0:01:00
 0:04:36
0:04:36
 0:12:57
0:12:57
 0:02:32
0:02:32
 0:20:21
0:20:21
 0:01:00
0:01:00