filmov
tv
VSEPR THEORY || Molecular geometry of CH4

Показать описание
This video explains Molecular geometry of CH4 molecule based on VSEPR theory.
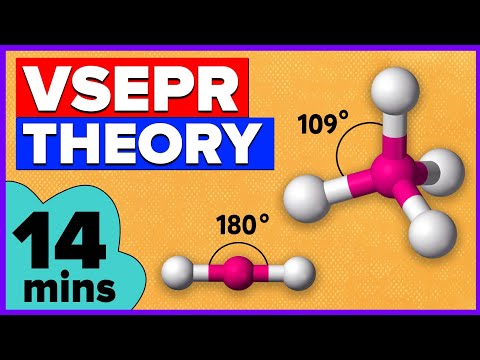
According to VSEPR theory, the shape of a covalent molecule depends upon the repulsion between the electron pairs in the valence shell of the central atom of the molecule. CH4 molecule does not contain lone pair on central atom.
Molecular geometry of CH4
Atomic Number of C-6
Electronic configuration-1s22s22p2
valence electrons-4.
Carbon share 4 of its electron with four H-atom and form 4 Bond pair.These 4 Bond pair electrons orient themselves and make the molecular geometry tetrahedral.
According to VSEPR theory, the shape of a covalent molecule depends upon the repulsion between the electron pairs in the valence shell of the central atom of the molecule. CH4 molecule does not contain lone pair on central atom.
Molecular geometry of CH4
Atomic Number of C-6
Electronic configuration-1s22s22p2
valence electrons-4.
Carbon share 4 of its electron with four H-atom and form 4 Bond pair.These 4 Bond pair electrons orient themselves and make the molecular geometry tetrahedral.
 0:06:31
0:06:31
 0:13:10
0:13:10
 0:10:23
0:10:23
 0:13:23
0:13:23
 0:11:01
0:11:01
 0:33:39
0:33:39
 0:05:38
0:05:38
 0:12:36
0:12:36
 0:00:13
0:00:13
 0:06:35
0:06:35
 0:12:48
0:12:48
 0:18:22
0:18:22
 0:14:58
0:14:58
 0:16:31
0:16:31
 0:06:16
0:06:16
 0:52:53
0:52:53
 0:08:39
0:08:39
 0:19:11
0:19:11
 0:14:04
0:14:04
 0:16:25
0:16:25
 0:12:29
0:12:29
 0:04:52
0:04:52
 0:07:28
0:07:28
 0:11:50
0:11:50