filmov
tv
9.1 VSEPR Theory and Molecular Shapes | General Chemistry

Показать описание
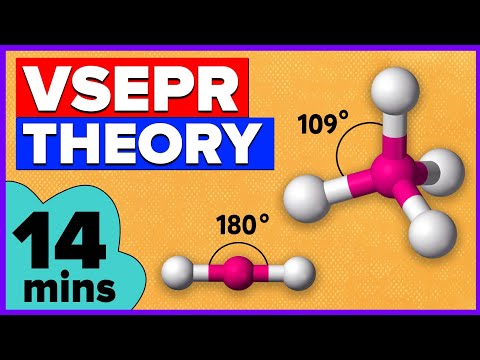
Chad provides a comprehensive lesson on VSEPR Theory and Molecular Geometry. The five fundamental Electron Domain Geometries (EDGs) including the bond angles are first covered: Linear, Trigonal Planar, Tetrahedral, Trigonal Bipyramidal, and Octahedral. Then the corresponding Molecular Geometries are covered being grouped by the number of electron domains with examples of each being presented. This includes the following:
2 Electron Domains: Linear
3 Electron Domains: Trigonal Planar & Bent
4 Electron Domains: Tetrahedral, Trigonal Pyramidal, & Bent
5 Electron Domains: Trigonal Bipyramidal, See-saw (aka Sawhorse), T-shaped, & Linear
6 Electron Domains: Octahedral, Square Pyramidal, & Square Planar
The effect of lone pairs and pi bonds reducing bond angles is also covered.
00:00 Lesson Introduction
00:44 VSEPR Theory, Electron Domain Geometry, and Molecular Geometry
12:09 Linear Molecular Geometry
13:55 3 Trigonal Planar Molecular Geometry (& Bent)
18:51 Tetrahedral Molecular Geometry (& Trigonal Pyramidal & Bent)
24:24 Trigonal Bipyramidal Molecular Geometry (& See-saw, T-shaped, & Linear)
29:54 Octahedral Molecular Geometry (& Square Pyramidal & Square Planar)
2 Electron Domains: Linear
3 Electron Domains: Trigonal Planar & Bent
4 Electron Domains: Tetrahedral, Trigonal Pyramidal, & Bent
5 Electron Domains: Trigonal Bipyramidal, See-saw (aka Sawhorse), T-shaped, & Linear
6 Electron Domains: Octahedral, Square Pyramidal, & Square Planar
The effect of lone pairs and pi bonds reducing bond angles is also covered.
00:00 Lesson Introduction
00:44 VSEPR Theory, Electron Domain Geometry, and Molecular Geometry
12:09 Linear Molecular Geometry
13:55 3 Trigonal Planar Molecular Geometry (& Bent)
18:51 Tetrahedral Molecular Geometry (& Trigonal Pyramidal & Bent)
24:24 Trigonal Bipyramidal Molecular Geometry (& See-saw, T-shaped, & Linear)
29:54 Octahedral Molecular Geometry (& Square Pyramidal & Square Planar)
Комментарии
 0:06:31
0:06:31
 0:33:39
0:33:39
 0:13:10
0:13:10
 0:10:23
0:10:23
 0:06:35
0:06:35
 0:05:38
0:05:38
 0:14:04
0:14:04
 0:14:06
0:14:06
 0:07:54
0:07:54
 0:09:57
0:09:57
 0:06:00
0:06:00
 0:11:01
0:11:01
 0:11:50
0:11:50
 0:14:47
0:14:47
 0:16:18
0:16:18
 0:14:58
0:14:58
 0:06:49
0:06:49
 0:21:29
0:21:29
 0:16:31
0:16:31
 0:00:51
0:00:51
 0:07:31
0:07:31
 0:09:51
0:09:51
 0:14:02
0:14:02
 1:16:48
1:16:48