filmov
tv
Lewis Diagrams and VSEPR Models

Показать описание
022 - Lewis Diagrams and VSEPR Models
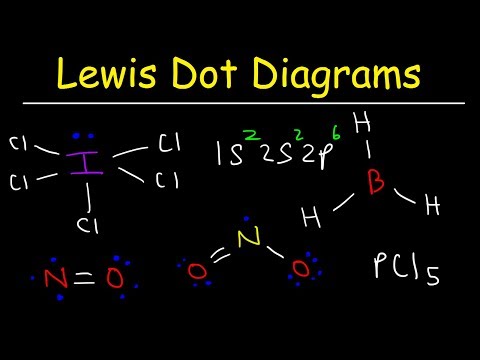
In this video Paul Andersen explains how you can use Lewis Diagrams and VSEPR Models to make predictions about molecules. The Lewis diagrams are a two-dimensional representations of covalent bonds and the VSEPR models show how the molecule could exist in three dimensional space. Pi bonding and odd valence electrons require an extension of this model.
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
In this video Paul Andersen explains how you can use Lewis Diagrams and VSEPR Models to make predictions about molecules. The Lewis diagrams are a two-dimensional representations of covalent bonds and the VSEPR models show how the molecule could exist in three dimensional space. Pi bonding and odd valence electrons require an extension of this model.
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
Lewis Diagrams and VSEPR Models
How To Draw Lewis Structures
VSEPR Theory and Molecular Geometry
Bonding Models and Lewis Structures: Crash Course Chemistry #24
VSEPR Theory - Basic Introduction
Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures
VSEPR Theory
Ch 9 Drawing VSEPR Structures
Lewis Diagrams and VSEPR Models
Lewis Structures and VSEPR Models
Molecular Geometry Made Easy: VSEPR Theory and How to Determine the Shape of a Molecule
VSEPR Megavideo: 36 Examples including Lewis Structure, Molecular Geometry, Intermolecular Forces
Lewis Dot Structures
VSEPR
How to.do Multiple Central Atom VSEPR Drawings
Molecular Geometry: Rules, Examples, and Practice
Molecular Geometry & VSEPR Theory - Basic Introduction
12. The Shapes of Molecules: VSEPR Theory
How to Draw Lewis Structures for Organic Chemistry
Quick Way to Memorize Molecular Geometry | Polarity | Angle | Hybridization | Ace That Exam
Lewis Dot, VSEPR Models and Polarity - for F2, CO2 (carbon dioxide) and CO32- (carbonate ion)
Exceptions To The Octet Rule - Lewis Dot Diagrams
Lewis Structure and Molecular Modeling Video 1
Lewis Structures Made Easy: Examples and Tricks for Drawing Lewis Dot Diagrams of Molecules
Комментарии
 0:12:29
0:12:29
 0:11:50
0:11:50
 0:06:31
0:06:31
 0:11:38
0:11:38
 0:13:10
0:13:10
 0:07:26
0:07:26
 0:05:38
0:05:38
 0:05:59
0:05:59
 0:12:29
0:12:29
 0:09:03
0:09:03
 0:13:23
0:13:23
 0:52:53
0:52:53
 0:04:41
0:04:41
 0:12:12
0:12:12
 0:09:50
0:09:50
 0:11:01
0:11:01
 0:10:23
0:10:23
 0:45:18
0:45:18
 0:10:37
0:10:37
 0:08:39
0:08:39
 0:13:43
0:13:43
 0:12:35
0:12:35
 0:02:38
0:02:38
 0:11:57
0:11:57