filmov
tv
internal energy and energy transfers - summary - GCSE Physics

Показать описание
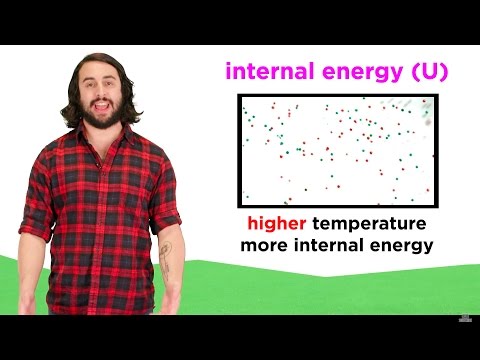
The energy stored by the particles in a system is called the internal energy.
Heating is defined as the movement of energy from an object with a higher temperature to an object with a lower temperature.
We use the particle model of matter to explain observations when energy is supplied to substances.
When the temperature of substance increases the kinetic energy of the particles increases, and when a substance changes state the potential energy of a particle increases.
Internal energy is the sum of the potential and kinetic energies of a substance.
Potential energy is due to an object’s position.
Potential energy of particles is higher when they are further apart.
Kinetic energy is due to an object’s speed.
Kinetic energy of particles is higher when they are moving faster.
Internal energy can be increased by heating or by doing work on a substance.
Other channels cover the content but GorillaPhysics will help you get the grade 9 or that A* at A Level.
Also, if you like GorillaPhysics and want to help me shape and expand my channel and resources, why not join my Patreon community and become part of Gary’s squad:
Student Amazon Prime! Free prime for six months and then half price from then on!
This would have been so useful as a student to get next day delivery on so many essentials, but also for the music, and amazon video you get included in prime as well. You might as well really! And you say thanks to GorillaPhysics as I get a small bounty when you use my link!
You can now support GorillaPhysics by buying your next educational purchase from Amazon at my store:
At Gorilla Physics we’re all about you understanding more, so you get more confident, then enjoy Physics more and then you’ll do better in your GCSE and A Level Physics exams.
Buy my book to help you memorise all the equations for GCSE Physics or Combined Science, for AQA, OCR A and B, Edexcel and Eduqas exam boards.
You can have the kindle edition for free on your phone with a free trial of kindle unlimited:
Otherwise check out these most popular playlists:
Heating is defined as the movement of energy from an object with a higher temperature to an object with a lower temperature.
We use the particle model of matter to explain observations when energy is supplied to substances.
When the temperature of substance increases the kinetic energy of the particles increases, and when a substance changes state the potential energy of a particle increases.
Internal energy is the sum of the potential and kinetic energies of a substance.
Potential energy is due to an object’s position.
Potential energy of particles is higher when they are further apart.
Kinetic energy is due to an object’s speed.
Kinetic energy of particles is higher when they are moving faster.
Internal energy can be increased by heating or by doing work on a substance.
Other channels cover the content but GorillaPhysics will help you get the grade 9 or that A* at A Level.
Also, if you like GorillaPhysics and want to help me shape and expand my channel and resources, why not join my Patreon community and become part of Gary’s squad:
Student Amazon Prime! Free prime for six months and then half price from then on!
This would have been so useful as a student to get next day delivery on so many essentials, but also for the music, and amazon video you get included in prime as well. You might as well really! And you say thanks to GorillaPhysics as I get a small bounty when you use my link!
You can now support GorillaPhysics by buying your next educational purchase from Amazon at my store:
At Gorilla Physics we’re all about you understanding more, so you get more confident, then enjoy Physics more and then you’ll do better in your GCSE and A Level Physics exams.
Buy my book to help you memorise all the equations for GCSE Physics or Combined Science, for AQA, OCR A and B, Edexcel and Eduqas exam boards.
You can have the kindle edition for free on your phone with a free trial of kindle unlimited:
Otherwise check out these most popular playlists:
Комментарии
 0:04:36
0:04:36
 0:03:48
0:03:48
 0:03:32
0:03:32
 0:05:44
0:05:44
 0:02:13
0:02:13
 0:05:10
0:05:10
 0:11:27
0:11:27
 0:25:54
0:25:54
 0:15:08
0:15:08
 0:05:45
0:05:45
 0:11:32
0:11:32
 0:11:52
0:11:52
 0:09:26
0:09:26
 0:01:34
0:01:34
 0:05:26
0:05:26
 0:06:26
0:06:26
 0:23:29
0:23:29
 0:11:16
0:11:16
 0:05:44
0:05:44
 0:07:15
0:07:15
 0:03:23
0:03:23
 0:00:09
0:00:09
 0:00:34
0:00:34
 0:03:07
0:03:07