filmov
tv
Class 9th – Polyatomic Ions | Atoms and Molecules | Tutorials Point

Показать описание
Polyatomic Ions
watch more videos at
Lecture By: Mrs. Priyanka Choudhary, Tutorials Point India Private Limited
watch more videos at
Lecture By: Mrs. Priyanka Choudhary, Tutorials Point India Private Limited
Polyatomic Ions in One Minute
How to memorize polyatomic ions?
How to Memorize The Polyatomic Ions - Formulas, Charges, Naming - Chemistry
Class 9th – Polyatomic Ions | Atoms and Molecules | Tutorials Point
How to Find Charge on Polyatomic ions? Easy Trick
Writing Formulas with Polyatomic Ions
Polyatomic Ion || in Hindi for Class 9
Monoatomic and Polyatomic Ions
What are polyatomic ions?Give example
How To Write Ionic Formulas With Polyatomic Ions
Atomicity - Monoatomic, Diatomic, Triatomic, Polyatomic Ions and Elements
Polyatomic Ions
EASY TRICK TO LEARN POLYATOMIC IONS
Easy way to Memorize PolyAtomic Ions
How to Memorize Polyatomic Ions [FAST]
Polyatomic Ions Grade 10
Polyatomic Ions |How to Memorize Polyatomic Ions | Tricks for Polyatomic Ions | Elementary Chemistry
BEST TRICK TO LEARN CHARGES ON IONS || IONS & CHARGE || NCERT SCIENCE CHAPTER 3 TABLE 3.6 || 9 C...
What is a Polyatomic Ion?
Ions Explained - Cations, Anions, Polyatomic Ions in Chemistry & Physics - [1-2-16]
How To Determine The Charge of Elements and Ions - Chemistry
How to memorize polyatomic ions|| How to easily find charge and formula of polyatomic anions
what are polyatomic ions? give examples - answer for 9th class ch3 q3
Class 9th – Chemical Formulae with Polyatomic Ions | Atoms and Molecules | Tutorials Point
Комментарии
 0:01:00
0:01:00
 0:15:47
0:15:47
 0:29:45
0:29:45
 0:14:44
0:14:44
 0:08:33
0:08:33
 0:11:21
0:11:21
 0:15:08
0:15:08
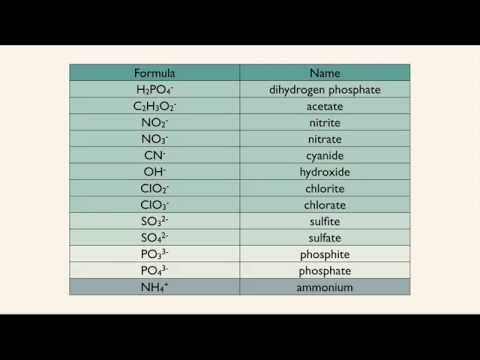 0:02:45
0:02:45
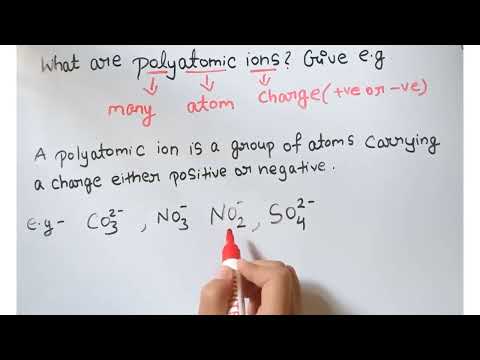 0:01:19
0:01:19
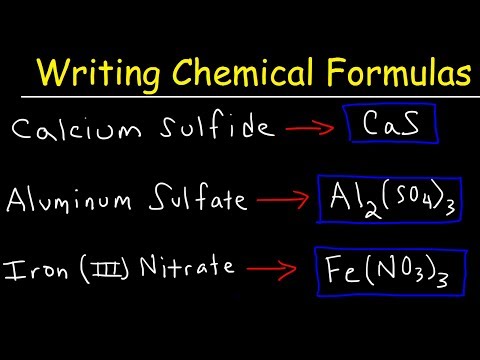 0:10:41
0:10:41
 0:05:07
0:05:07
 0:02:04
0:02:04
 0:02:05
0:02:05
 0:03:22
0:03:22
 0:17:57
0:17:57
 0:12:39
0:12:39
 0:10:39
0:10:39
 0:06:50
0:06:50
 0:06:58
0:06:58
 0:34:07
0:34:07
 0:19:12
0:19:12
 0:18:44
0:18:44
 0:02:28
0:02:28
 0:07:28
0:07:28