filmov
tv
Polyatomic Ions in One Minute

Показать описание
In this video, you will learn about the different polyatomic ions. We'll also look at the most important polyatomic ions to memorize as well as how to use a table of ions (if you are allowed to).
Polyatomic ions are groups of atoms that carry a charge and exist as a single unit. They are commonly found in ionic compounds, and it is essential to know their names, formulas, and charges to be successful chemistry.
We will discuss the most common polyatomic ions, including their names, formulas, and charges. Additionally, we'll look at how to use a table of polyatomic ions to aid in memorization and recall.
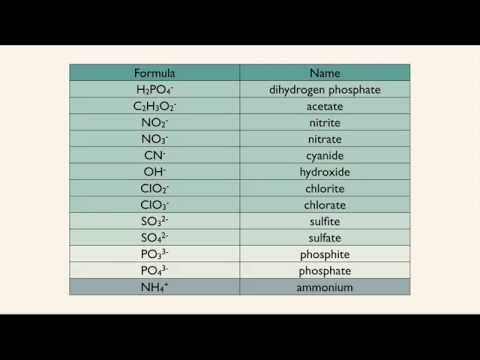
Polyatomic ion table shown in video:
Helpful videos:
Naming Compounds with Polyatomic Ions:
Polyatomic ions are groups of atoms that carry a charge and exist as a single unit. They are commonly found in ionic compounds, and it is essential to know their names, formulas, and charges to be successful chemistry.
We will discuss the most common polyatomic ions, including their names, formulas, and charges. Additionally, we'll look at how to use a table of polyatomic ions to aid in memorization and recall.
Polyatomic ion table shown in video:
Helpful videos:
Naming Compounds with Polyatomic Ions:
Polyatomic Ions in One Minute
How to Memorize The Polyatomic Ions - Formulas, Charges, Naming - Chemistry
How to memorize polyatomic ions?
Understanding the Polyatomic Ions In Under 10 Minutes
How to Memorize Polyatomic Ions [FAST]
Memorizing and Using the List of Polyatomic Ions
Polyatomic Ions Song (God' Plan by Drake)
Polyatomic Ions
Gen Chem Study Session with Dr. Taylor - September 30, 2024
List of Polyatomic Ions
Quick trick to memorize polyatomic ions for AP chemistry
Easy way to Memorize PolyAtomic Ions
Monoatomic and Polyatomic Ions
5 Minute Fun: Polyatomic Ions
A satisfying chemical reaction
How to Find Charge on Polyatomic ions? Easy Trick
Song Of chemistry 😂😂 #science #chemistry #songs
Polyatomic ion chart neet #basics #chemistry
Can you have two different polyatomic ions in a compound?
Polyatomic Ions Song
Common Polyatomic ions IIT JEE NEET
What are Polyatomic Ions? (Polyatomic Ions Examples)
how to remember polyatomic ions
Polyatomic Ions
Комментарии
 0:01:00
0:01:00
 0:29:45
0:29:45
 0:15:47
0:15:47
 0:08:42
0:08:42
 0:17:57
0:17:57
 0:05:58
0:05:58
 0:03:03
0:03:03
 0:02:04
0:02:04
 2:01:41
2:01:41
 0:01:41
0:01:41
 0:05:05
0:05:05
 0:03:22
0:03:22
 0:02:45
0:02:45
 0:06:32
0:06:32
 0:00:19
0:00:19
 0:08:33
0:08:33
 0:00:14
0:00:14
 0:00:07
0:00:07
 0:01:57
0:01:57
 0:01:36
0:01:36
 0:00:06
0:00:06
 0:08:02
0:08:02
 0:05:58
0:05:58
 0:07:41
0:07:41