filmov
tv
Monoatomic and Polyatomic Ions

Показать описание
learn about monoatomic and polyatomic ions and how they are located and named.
Monoatomic and Polyatomic Ions
How to Memorize The Polyatomic Ions - Formulas, Charges, Naming - Chemistry
Atomicity - Monoatomic, Diatomic, Triatomic, Polyatomic Ions and Elements
Naming Compounds with Polyatomic Ions
Polyatomic Ions in One Minute
Polyatomic Ions Song
Naming Monoatomic Ions
Monatomic Ions
Atoms & Molecules | Class 9 Science Chapter 3 | Part - 3 | Modern symbols | Important Explanatio...
Memorize Polyatomic Ions to Hotline Bling Song (AP Chemistry Fate)
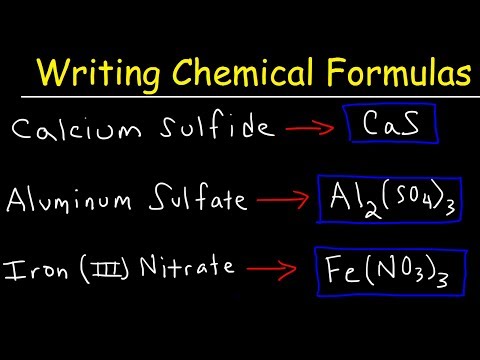
Writing Formulas with Polyatomic Ions
Monoatomic and polyatomic ions | Fundamentals of Chemistry | LabTurtle
Common polyatomic ions | Atoms, compounds, and ions | Chemistry | Khan Academy
polyatomic and monatomic ions podcast
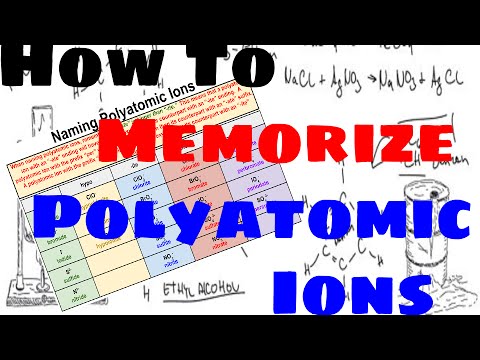
How to memorize polyatomic ions?
What is a Polyatomic Ion?
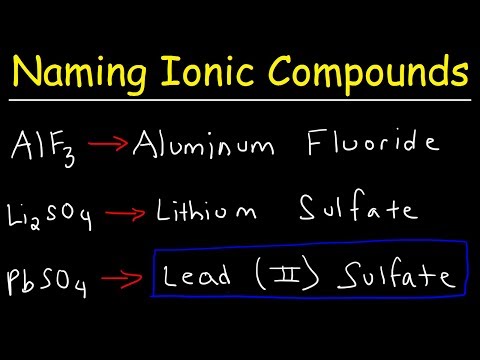
Naming Ionic Compounds
How To Write Ionic Formulas With Polyatomic Ions
How to Memorize and Name Polyatomic Ions
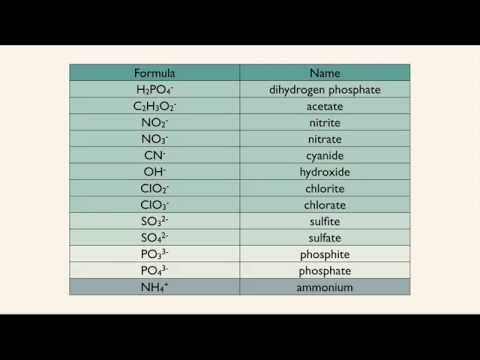
MONOATOMIC AND POLYATOMIC IONS
Polyatomic Ions Grade 10
Nitrate Nitrite Nitride | ate ite ide | Monoatomic and Polyatomic ions - Dr K
Lecture 10.1 Monoatomic and Polyatomic Ions
How To Name Ionic Compounds With Transition Metals
Комментарии
 0:02:45
0:02:45
 0:29:45
0:29:45
 0:05:07
0:05:07
 0:05:26
0:05:26
 0:01:00
0:01:00
 0:01:36
0:01:36
 0:12:31
0:12:31
 0:03:39
0:03:39
 0:21:57
0:21:57
 0:04:01
0:04:01
 0:11:21
0:11:21
 0:08:02
0:08:02
 0:05:48
0:05:48
 0:03:02
0:03:02
 0:15:47
0:15:47
 0:06:58
0:06:58
 0:05:44
0:05:44
 0:10:41
0:10:41
 0:07:00
0:07:00
 0:02:25
0:02:25
 0:12:39
0:12:39
 0:04:27
0:04:27
 0:11:36
0:11:36
 0:13:33
0:13:33