filmov
tv
11.1 Intermolecular Forces | General Chemistry

Показать описание
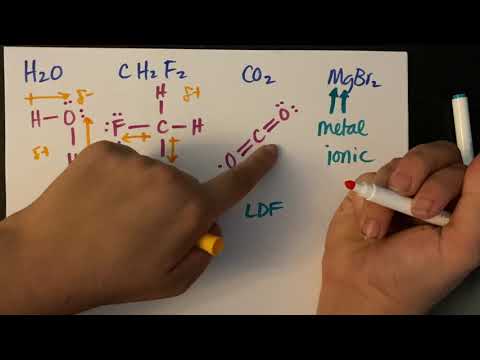
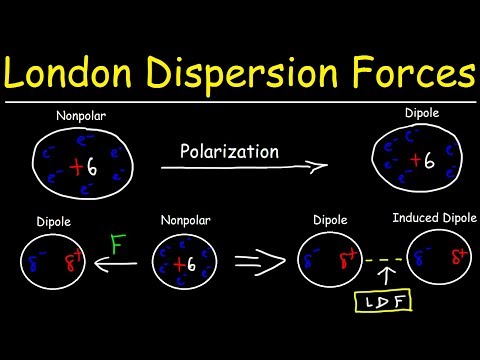
Chad provides a comprehensive lesson on Intermolecular Forces and how they affect the bulk properties of liquids and solids. The lesson includes definitions and descriptions of Hydrogen Bonding, Dipole-Dipole Forces, London Dispersion Forces, and Ion-Dipole Forces. The lesson also expounds on how greater intermolecular forces lead to higher boiling points, higher melting points, higher enthalpy of vaporization and enthalpy of fusion, greater viscosity, greater surface tension, higher critical temperature and pressure, and lower vapor pressure. Finally, four examples of ranking intermolecular forces are worked out for the student.
00:00 Lesson Introduction
00:51 What are Intermolecular Forces?
03:00 Dipole-Dipole Forces
05:35 Hydrogen Bonding
10:33 London Dispersion Forces
14:10 Ion-Dipole Forces
17:13 Intermolecular Forces and Properties of Liquids
23:07 Vapor Pressure and Boiling Point
26:14 Ranking Intermolecular Forces Example #1
27:54 Ranking Intermolecular Forces Example #2
30:12 Ranking Intermolecular Forces Example #3
31:28 Ranking Intermolecular Forces Example #4
00:00 Lesson Introduction
00:51 What are Intermolecular Forces?
03:00 Dipole-Dipole Forces
05:35 Hydrogen Bonding
10:33 London Dispersion Forces
14:10 Ion-Dipole Forces
17:13 Intermolecular Forces and Properties of Liquids
23:07 Vapor Pressure and Boiling Point
26:14 Ranking Intermolecular Forces Example #1
27:54 Ranking Intermolecular Forces Example #2
30:12 Ranking Intermolecular Forces Example #3
31:28 Ranking Intermolecular Forces Example #4
Комментарии
 0:35:58
0:35:58
 0:10:54
0:10:54
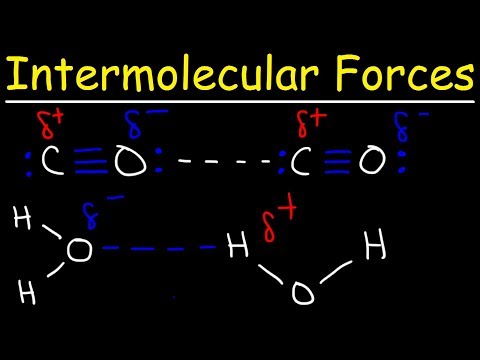 0:10:40
0:10:40
 0:08:07
0:08:07
 0:05:50
0:05:50
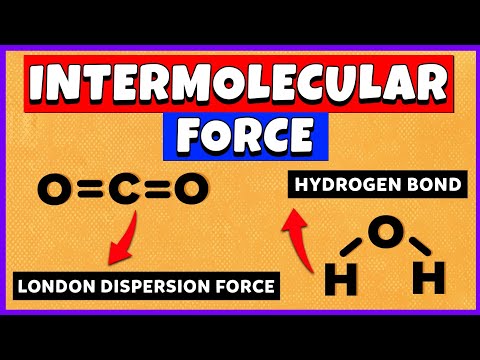 0:08:05
0:08:05
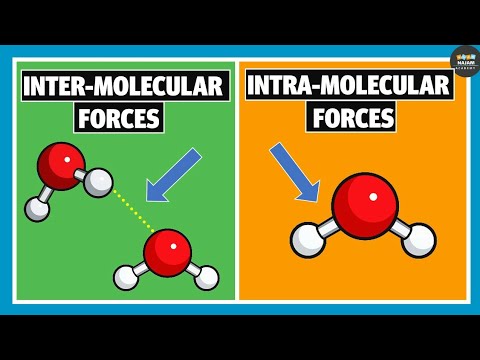 0:06:53
0:06:53
 0:33:34
0:33:34
 1:02:03
1:02:03
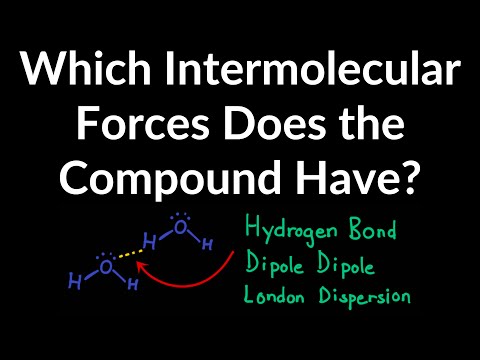 0:05:37
0:05:37
 0:02:51
0:02:51
 0:38:14
0:38:14
 0:55:46
0:55:46
 1:01:14
1:01:14
 0:09:33
0:09:33
 0:04:23
0:04:23
 0:09:18
0:09:18
 0:08:39
0:08:39
 0:11:17
0:11:17
 0:05:20
0:05:20
 0:09:38
0:09:38
 0:40:57
0:40:57
 0:06:17
0:06:17
 0:21:14
0:21:14