filmov
tv
London Dispersion Forces & Temporary Dipole - Induced Dipole Interactions - Intermolecular Forces

Показать описание
This chemistry video tutorial provides a basic introduction into london dispersion forces also known van der waals forces. London dispersion forces arises from the electrostatic interactions between temporary dipoles and induced dipoles. A dipole is a polarized particle that contains a separation of charge - one part of the particle is partially positive and the other part is partially positive. Polar molecules contain permanent dipoles. Nonpolar molecules do not usually contain a dipole moment but can become a temporary dipole due to the distortion of the electron cloud. Atoms and molecules with a large number of electrons are highly polarizable, that is, they have a higher probability of turning into a temporary dipole. A dipole can cause another molecule to turn into a temporary dipole. This is known as an induced dipole. The interactions between temporary instantaneous dipoles and induced dipoles are known as van der waals interactions or london dispersion forces. Large molecules have a high amount of london dispersion forces and therefore have higher boiling points than smaller molecules. Boiling point is directly related to the amount of van der waal interactions among molecules.
Lewis Structures - Mega Review:
Sigma and Pi Bonding:
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
_______________________________
Hydrogen Bonding:
London Dispersion Forces:
Ion Dipole Forces:
Bragg's Equation For X-Ray Diffraction:
Molecular & Network Covalent Solids:
_______________________________
Metallic Bonding:
Metal Alloys:
Diamond Vs Graphite:
Semiconductors:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Lewis Structures - Mega Review:
Sigma and Pi Bonding:
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
_______________________________
Hydrogen Bonding:
London Dispersion Forces:
Ion Dipole Forces:
Bragg's Equation For X-Ray Diffraction:
Molecular & Network Covalent Solids:
_______________________________
Metallic Bonding:
Metal Alloys:
Diamond Vs Graphite:
Semiconductors:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
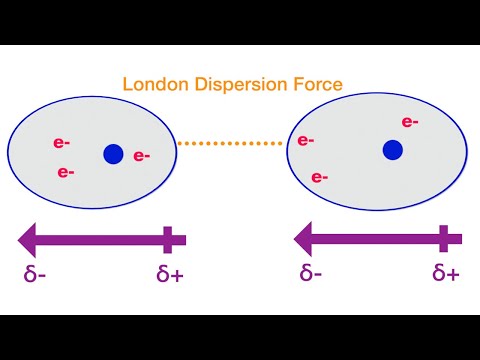 0:00:22
0:00:22
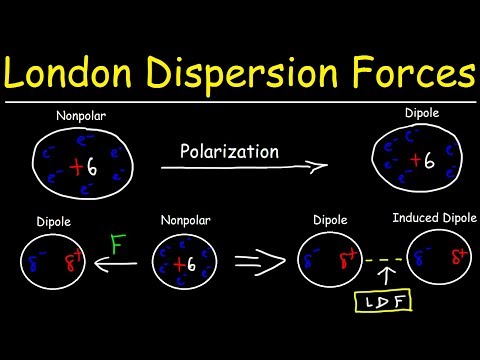 0:11:17
0:11:17
 0:06:56
0:06:56
 0:01:36
0:01:36
 0:08:39
0:08:39
 0:00:19
0:00:19
 0:05:02
0:05:02
 0:10:54
0:10:54
 0:10:36
0:10:36
 0:01:27
0:01:27
 0:00:40
0:00:40
 0:09:38
0:09:38
 0:16:36
0:16:36
 0:09:25
0:09:25
 0:04:33
0:04:33
 0:08:30
0:08:30
 0:07:20
0:07:20
 0:00:52
0:00:52
 0:01:43
0:01:43
 0:04:55
0:04:55
 0:03:23
0:03:23
 0:13:32
0:13:32
 0:05:04
0:05:04
 0:09:59
0:09:59