filmov
tv
Osmosis and Tonicity-Hypertonic, Hypotonic and Isotonic Solutions-Osmosis with Raw and Boiled Potato
Показать описание
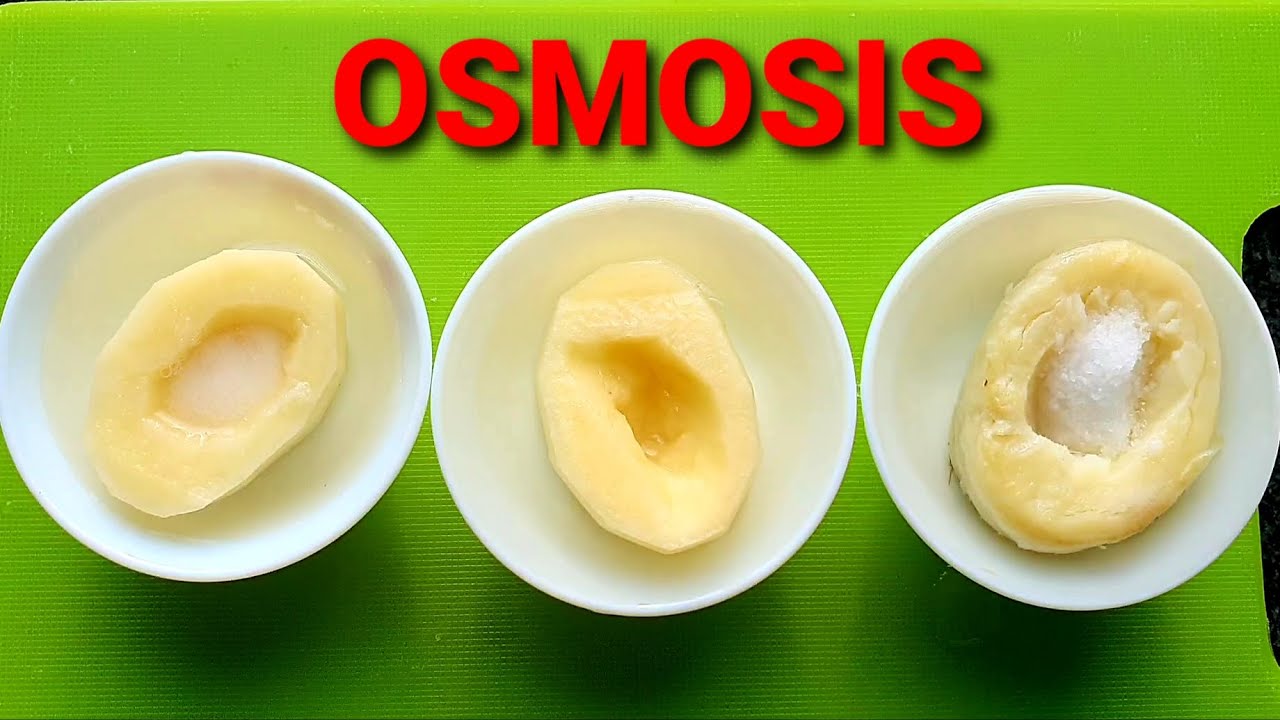
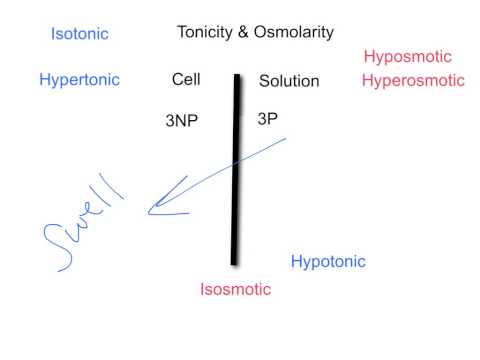
Tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a semipermeable cell membrane. In other words, tonicity is the relative concentration of solutes dissolved in solution which determine the direction and extent of diffusion.
Osmosis is the spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides.
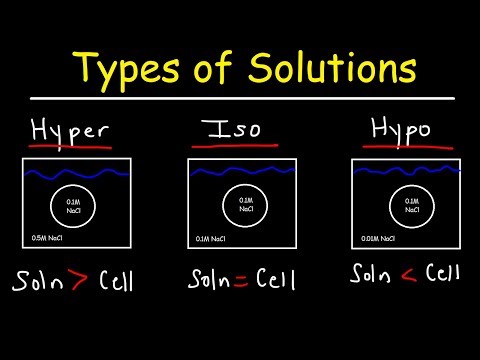
A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to the cytosol. Due to osmotic pressure, water diffuses into the cell, and the cell often appears turgid, or bloated. This is called Endosmosis.
If the medium surrounding the cell has a lower water concentration than inside the cell, i.e., if the solution is highly concentrated, then the cell will lose water through osmosis. This is called Exosmosis. Such concentrated solution is called Hypertonic solution.
If the medium surrounding the cell is of exactly the same water concentration as inside the cell, there will be no net movement of water across membrane resulting in no change in size of cell. Such solution is called Isotonic solution.
#CuriosityVelocity
#Biology
#Tonicity
#OsmosisAndDiffusion
#hypertonicsolutions
#OsmosisWithPotato
#hypertonicity
#hypotonicandhypertonic
hypotonic hypertonic and isotonic,
hypotonic solution,
hypotonic vs hypertonic
osmosis experiment
osmosis and tonicity
osmosis biology
Osmosis is the spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides.
A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to the cytosol. Due to osmotic pressure, water diffuses into the cell, and the cell often appears turgid, or bloated. This is called Endosmosis.
If the medium surrounding the cell has a lower water concentration than inside the cell, i.e., if the solution is highly concentrated, then the cell will lose water through osmosis. This is called Exosmosis. Such concentrated solution is called Hypertonic solution.
If the medium surrounding the cell is of exactly the same water concentration as inside the cell, there will be no net movement of water across membrane resulting in no change in size of cell. Such solution is called Isotonic solution.
#CuriosityVelocity
#Biology
#Tonicity
#OsmosisAndDiffusion
#hypertonicsolutions
#OsmosisWithPotato
#hypertonicity
#hypotonicandhypertonic
hypotonic hypertonic and isotonic,
hypotonic solution,
hypotonic vs hypertonic
osmosis experiment
osmosis and tonicity
osmosis biology
Комментарии
 0:02:24
0:02:24
 0:04:46
0:04:46
 0:06:29
0:06:29
 0:01:52
0:01:52
 0:05:04
0:05:04
 0:13:09
0:13:09
 0:03:24
0:03:24
 0:02:52
0:02:52
 0:14:43
0:14:43
 0:02:23
0:02:23
 0:01:09
0:01:09
 0:02:29
0:02:29
 0:09:50
0:09:50
 0:11:55
0:11:55
 0:09:20
0:09:20
 0:05:40
0:05:40
 0:07:02
0:07:02
 0:16:26
0:16:26
 0:04:28
0:04:28
 0:00:52
0:00:52
 0:03:21
0:03:21
 0:01:00
0:01:00
 0:04:08
0:04:08
 0:11:19
0:11:19