filmov
tv
Osmolality, Osmolarity, and Tonicity - Physiology, Biology, and Chemistry
Показать описание
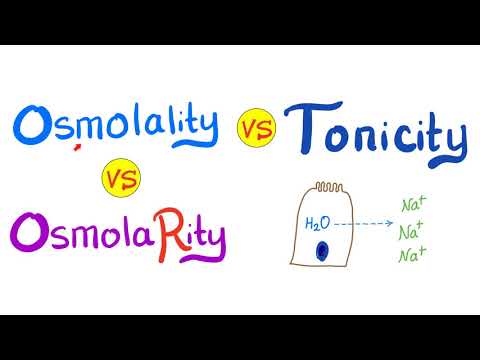
Osmolality, Osmolarity, and Tonicity.
Osmosis is a special type of simple diffusion (specifically for water). Osmosis is the movement of water from high concentration of water (low concentration of solute) into low concentration of water (high concentration of solute).
What's the difference between Mitosis and Meiosis?
- With Picmonic, get your life back by studying less and remembering more. Medical and Nursing students say that Picmonic is the most comprehensive and effective way to bridge learning and test prep...
Disclaimer: I use affiliate links....
Osmosis is a special type of simple diffusion (specifically for water). Osmosis is the movement of water from high concentration of water (low concentration of solute) into low concentration of water (high concentration of solute).
What's the difference between Mitosis and Meiosis?
- With Picmonic, get your life back by studying less and remembering more. Medical and Nursing students say that Picmonic is the most comprehensive and effective way to bridge learning and test prep...
Disclaimer: I use affiliate links....
Osmolality, Osmolarity, and Tonicity - Physiology, Biology, and Chemistry
LPA 1B - Osmolarity vs Tonicity
Chemistry Basics: Osmolarity, Osmolality and Tonicity
Osmosis | Osmolarity | Osmotic Equilibrium | Transport Across the Cell Membrane | Cell Physiology
Osmosis and Tonicity
Osmolality vs Osmolarity (with a mnemonic) - Physiology and Chemistry
Osmosis Explained: Solutes and Osmolarity | What is Osmosis Nursing School Review NCLEX
Osmolality vs Tonicity
Tonicity
Tonicity
Tonicity & Osmolarity
Hypertonic, Hypotonic and Isotonic Solutions!
Fluid & Hormones | IV Fluids (Isotonic, Hypotonic, & Hypertonic)
Serum Osmolality - What is the Osmolarity of your plasma?
Tonicity & Osmolarity
Hyponatremia: Tonicity and Osmolality
IV Fluid Types & Uses Nursing IV Therapy: Isotonic, Hypertonic, Hypotonic Solutions Tonicity NCL...
Osmolarity, Osmolality and Tonicity
Osmolarity vs Osmolality
Osmolarity and Osmolality versus Tonicity - Explained!
PHYSIOLOGY; CONCENTRATION OF SOLUTIONS; PART 3; TONICITY & OSMOLARITY by Professor Fink
OSMOSIS EXPLAINED! What is osmolarity? What are osmoles?
Body Fluids: Intracellular Fluids, and Extracellular Fluids, Osmolarity Vs Osmolality Vs Tonicity
Fluid Therapy 2: Solutes,Osmolality and Tonicity
Комментарии
 0:03:24
0:03:24
 0:09:44
0:09:44
 0:06:55
0:06:55
 0:02:24
0:02:24
 0:06:38
0:06:38
 0:03:21
0:03:21
 0:21:10
0:21:10
 0:13:09
0:13:09
 0:05:04
0:05:04
 0:08:55
0:08:55
 0:04:46
0:04:46
 0:02:52
0:02:52
 0:24:50
0:24:50
 0:05:40
0:05:40
 0:12:28
0:12:28
 0:16:26
0:16:26
 0:19:21
0:19:21
 0:02:40
0:02:40
 0:09:29
0:09:29
 0:32:14
0:32:14
 0:08:35
0:08:35
 0:11:39
0:11:39
 0:12:50
0:12:50