filmov
tv
LPA 1B - Osmolarity vs Tonicity

Показать описание
LPA 1B - Osmolarity vs Tonicity
Osmolality vs Osmolarity (with a mnemonic) - Physiology and Chemistry
osmolarity vs tonicity
Osmolality, Osmolarity, and Tonicity - Physiology, Biology, and Chemistry
JABSOM MD1 | Intro to Cell Physiology | Osmolality & Osmolarity
Calculated Serum Osmolality & Osmolar Gap
Osmolarity vs Tonicity
Osmosis | Osmolarity | Osmotic Equilibrium | Transport Across the Cell Membrane | Cell Physiology
LPA 1A - Osmosis
LPA 1C - Osmolarity Practice Problems
Serum Osmolality - What is the Osmolarity of your plasma?
Total osmolarity VS Effective osmolarity (tonicity)
molarity vs osmolarity vs tonicity
Body Fluids: Intracellular Fluids, and Extracellular Fluids, Osmolarity Vs Osmolality Vs Tonicity
Osmolality vs Osmolarity
Osmolarity vs. Water Potential
Osmolarity Vs Osmolality Vs Tonicity
Osmolality vs Tonicity
What is serum osmolarity?
Osmosis Explained: Solutes and Osmolarity | What is Osmosis Nursing School Review NCLEX
Osmolarity vs Osmolality
Tonicity and Osmolarity
Renal physiology 16 | Isosmotic vs Isotonic | Hypotonic vs Hypo-osmotic | Hypertonic Hyperosmotic
Osmolarity vs Tonicity
Комментарии
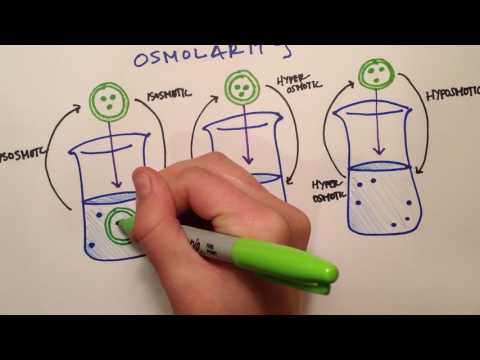 0:03:24
0:03:24
 0:06:38
0:06:38
 0:11:25
0:11:25
 0:11:19
0:11:19
 0:05:00
0:05:00
 0:01:00
0:01:00
 0:04:42
0:04:42
 0:06:55
0:06:55
 0:04:14
0:04:14
 0:08:27
0:08:27
 0:24:50
0:24:50
 1:06:43
1:06:43
 0:04:07
0:04:07
 0:11:39
0:11:39
 0:00:55
0:00:55
 0:01:52
0:01:52
 0:08:58
0:08:58
 0:21:10
0:21:10
 0:00:36
0:00:36
 0:03:21
0:03:21
 0:02:40
0:02:40
 0:26:49
0:26:49
 0:09:00
0:09:00
 0:15:11
0:15:11