filmov
tv
Defining Acids and Bases with Arrhenius, Bronsted-Lowry and Lewis

Показать описание
There are three common definitions of acids and bases. We will start with the most narrow of the definitions, Arrhenius and move to Lewis's very broad definition. Which of the three is most commonly used? Why, Bronsted-Lowry, of course!
What Is The Bronsted Lowry Theory | Acids, Bases & Alkali's | Chemistry | FuseSchool
GCSE Chemistry - Acids and Bases #34
Acid and Base Definitions | Arrhenius, Bronsted-Lowry, and Lewis
Acids and Bases - Basic Introduction - Chemistry
Acids and Bases, pH and pOH
What is a base in Chemistry? Acids and Bases
Brønsted–Lowry acids and bases | Chemical reactions | AP Chemistry | Khan Academy
Arrhenius definition of acids and bases | Biology | Khan Academy
A Level | Live Class 44 | Organic Chemistry | Acids abd Bases | Acids Comparison | +92 323 509 4443
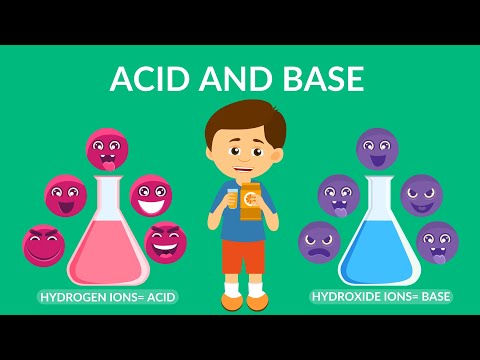
Acid and Base | Acids, Bases & pH | Video for Kids
Defining Acids and Bases with Arrhenius, Bronsted-Lowry and Lewis
Acids and Bases - Basic Introduction - Organic Chemistry
Grade 11 Acids and Bases: Type of reactions Introduction | Defining acids and bases
Acid/Base Definitions
General Chemistry | Acids & Bases
16.1 Introduction to Acids and Bases | General Chemistry
Conjugate Acids & Bases | Acids, Bases & Alkali's | Chemistry | FuseSchool
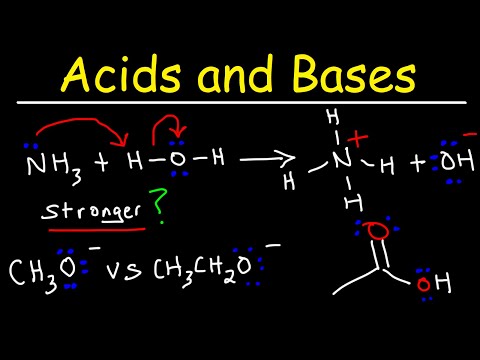
Lewis Acids and Bases
Defining Acids and Bases with Arrhenius and Bronsted Lowry
Acid Base Concepts (Arrhenius, Lowry-Bronsted & Lewis) in Urdu/Hindi
Acids and Bases
How to Memorize Strong Bases | Trick to Learn Strong Bases #shorts #reels #chemistry
Bronsted Lowry Acids and Bases | Chemistry
Define Acid l What is Acid? l The definitions of acid?
Комментарии
 0:03:56
0:03:56
 0:04:37
0:04:37
 0:02:06
0:02:06
 0:58:42
0:58:42
 0:09:01
0:09:01
 0:02:43
0:02:43
 0:08:56
0:08:56
 0:07:49
0:07:49
 0:44:40
0:44:40
 0:03:13
0:03:13
 0:05:37
0:05:37
 0:29:55
0:29:55
 0:15:07
0:15:07
 0:08:54
0:08:54
 0:33:17
0:33:17
 0:32:34
0:32:34
 0:03:46
0:03:46
 0:06:22
0:06:22
 0:03:23
0:03:23
 0:06:53
0:06:53
 0:00:15
0:00:15
 0:00:45
0:00:45
 0:05:31
0:05:31
 0:02:30
0:02:30