filmov
tv
Lewis Acids and Bases

Показать описание
This organic chemistry video tutorial provides a basic introduction into lewis acids and bases. It explains how to predict the products of a lewis acid-base reaction.
Acids and Bases - Basic Intro:
Lewis Acids and Bases:
Nucleophiles and Electrophiles:
Hydrocarbons:
Constitutional Isomers:
_______________________________
IUPAC Nomenclature of Alkanes:
Naming Cycloalkanes:
Naming Bicyclic Compounds:
Naming Ethers:
Naming Alcohols:
Naming Alkyl Halides:
________________________________
Naming Amines:
Van Der Waal Forces:
Boiling Point of Organic Compounds:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
Acids and Bases - Basic Intro:
Lewis Acids and Bases:
Nucleophiles and Electrophiles:
Hydrocarbons:
Constitutional Isomers:
_______________________________
IUPAC Nomenclature of Alkanes:
Naming Cycloalkanes:
Naming Bicyclic Compounds:
Naming Ethers:
Naming Alcohols:
Naming Alkyl Halides:
________________________________
Naming Amines:
Van Der Waal Forces:
Boiling Point of Organic Compounds:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
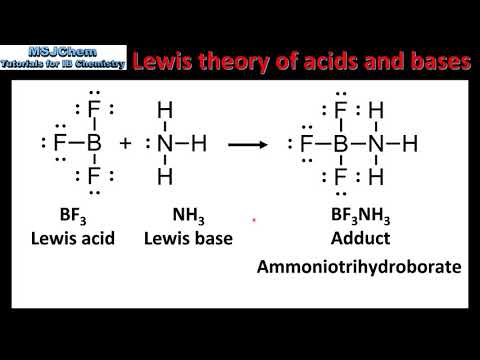
Lewis Acids and Bases
How to Identify Lewis Acid and Lewis Base Shortcut, Practice Problems, Examples, Explained
A3Academy: Lewis Acids and Bases
Lewis Acids and Bases
What Are Lewis Acids and Bases? Ft. Professor Dave
Chem163 Lewis Acids and Bases (15.12)
Acid and Base Definitions | Arrhenius, Bronsted-Lowry, and Lewis
Identifying Lewis Acids and Lewis Bases 001
How to Identify Lewis Acids and Lewis Base
Chapter Acid Bases & Salts - Lewis Concept of Acid & Bases by Tariq Pathan
Lewis Acids and Lewis Bases
Lewis Theory | Lewis acids and bases| How to identify Lewis Acids and Bases?
How to identify Lewis acids and bases #lewis #lewisacid #lewisbase #chemistry #shorts #shortvideo
Acids and Bases, pH and pOH
Lewis theory | lewis concept of acids and bases | theories of acids and bases | acids and bases
Lewis Acids and Bases
Lewis Acids and Bases
R3.4.6 What are Lewis Acids and Bases? [HL IB Chemistry]
Class 10 Chemistry Chapter 10 - Lewis Concept of Acids and Bases - 10th Class Chemistry Chapter 2
Lewis Concept of Acid and bases | 10th class chemistry | ch.no.10
ALEKS - Identifying Lewis Acids and Bases in Reactions
How to Remember Lewis Acid & Lewis Base #shorts #magnetbrains
R3.4.6 / R3.4.7 Lewis theory of acids and bases (HL)
Trick to find Lewis acid and Lewis base | Equilibrium |Class-11th | IIT-JEE, NEET CHEMISTRY
Комментарии
 0:06:22
0:06:22
 0:03:00
0:03:00
 0:03:36
0:03:36
 0:16:46
0:16:46
 0:03:29
0:03:29
 0:05:11
0:05:11
 0:02:06
0:02:06
 0:03:09
0:03:09
 0:02:10
0:02:10
 0:11:17
0:11:17
 0:05:01
0:05:01
 0:03:26
0:03:26
 0:01:01
0:01:01
 0:09:01
0:09:01
 0:02:22
0:02:22
 0:04:33
0:04:33
 0:04:53
0:04:53
 0:04:21
0:04:21
 0:28:03
0:28:03
 0:15:53
0:15:53
 0:06:39
0:06:39
 0:00:52
0:00:52
 0:04:10
0:04:10
 0:06:55
0:06:55