filmov
tv
Calculate Percent Yield with Ideal Stoichiometry - Practice - 1

Показать описание
When 50.0 g of silicon dioxide is heated with an excess of carbon, 32.2 g of silicon carbide is produced. SiO2(s) + 3C(s) -- SiC(s) + 2CO(g)
a. What is the percent yield of this reaction?
b. How many grams of CO gas are produced?
Teachers Pay Teachers Practice Worksheets:
Percent Yield Practice Worksheet 1.0:
Answer Key:
Question is based off Pearson Chemistry Chapter 12 Assessment Question 61.
a. What is the percent yield of this reaction?
b. How many grams of CO gas are produced?
Teachers Pay Teachers Practice Worksheets:
Percent Yield Practice Worksheet 1.0:
Answer Key:
Question is based off Pearson Chemistry Chapter 12 Assessment Question 61.
 0:08:58
0:08:58
 0:06:24
0:06:24
 0:07:54
0:07:54
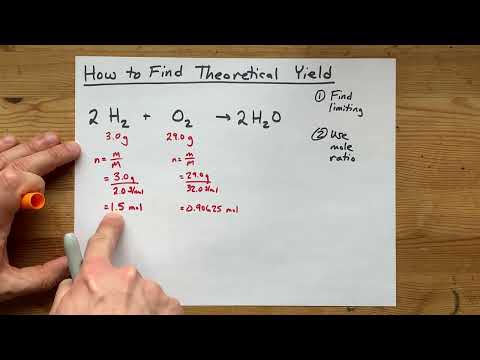 0:05:22
0:05:22
 0:07:45
0:07:45
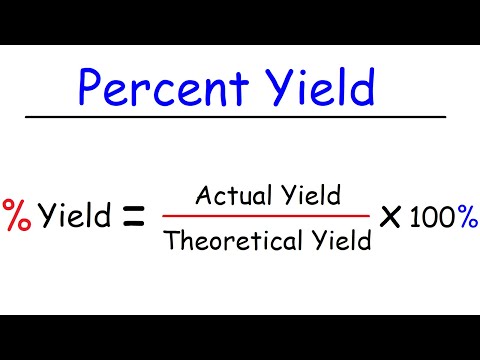 0:11:03
0:11:03
 0:03:50
0:03:50
 0:04:35
0:04:35
 0:04:13
0:04:13
 0:09:33
0:09:33
 0:04:07
0:04:07
 0:09:30
0:09:30
 0:05:50
0:05:50
 0:16:57
0:16:57
 0:21:30
0:21:30
 0:11:52
0:11:52
 0:05:04
0:05:04
 0:16:27
0:16:27
 0:30:15
0:30:15
 0:05:51
0:05:51
 0:08:49
0:08:49
 0:03:50
0:03:50
 0:10:00
0:10:00
 0:03:54
0:03:54