filmov
tv
Calculate Percent Yield with Ideal Stoichiometry - Practice - 2

Показать описание
If the reaction below proceeds with a 96.8% yield, how many kilograms of CaSO4 are formed when 5.24 kg SO2 reacts with an excess of CaCO3 and O2?
2CaCO3(s) + 2SO2(g) + O2(g) = 2CaSO4(s) + 2CO2(g)
Teachers Pay Teachers Practice Worksheets:
Percent Yield Practice Worksheet 1.0
Answer Key:
Percent Yield Practice Worksheet 2.0
Answer Key:
Question is based off Pearson Chemistry Chapter 12 Assessment Question 62
2CaCO3(s) + 2SO2(g) + O2(g) = 2CaSO4(s) + 2CO2(g)
Teachers Pay Teachers Practice Worksheets:
Percent Yield Practice Worksheet 1.0
Answer Key:
Percent Yield Practice Worksheet 2.0
Answer Key:
Question is based off Pearson Chemistry Chapter 12 Assessment Question 62
 0:08:58
0:08:58
 0:06:24
0:06:24
 0:07:54
0:07:54
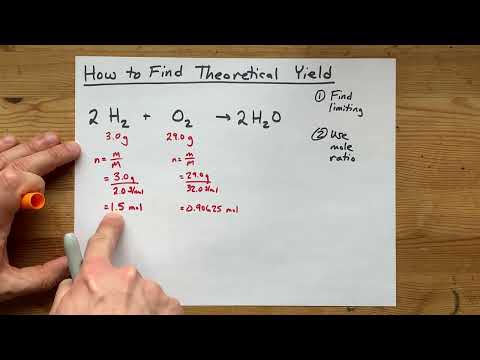 0:05:22
0:05:22
 0:07:45
0:07:45
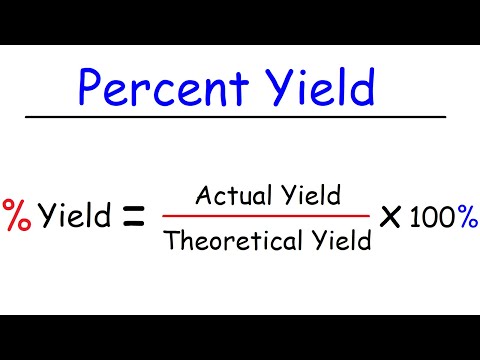 0:11:03
0:11:03
 0:04:35
0:04:35
 0:03:50
0:03:50
 0:09:33
0:09:33
 0:11:52
0:11:52
 0:21:30
0:21:30
 0:04:13
0:04:13
 0:09:30
0:09:30
 0:16:57
0:16:57
 0:03:54
0:03:54
 0:06:17
0:06:17
 0:03:50
0:03:50
 0:16:33
0:16:33
 0:08:49
0:08:49
 0:03:25
0:03:25
 0:03:16
0:03:16
 0:21:42
0:21:42
 0:07:32
0:07:32
 0:02:46
0:02:46