filmov
tv
IB Chemistry Topic 4.4 Intermolecular forces

Показать описание
IB Chemistry Topic 4.4 Intermolecular forces
The different types of intermolecular forces - London dispersion forces, dipole-dipole forces, and hydrogen bonding. How the different intermolecular forces cause different physical properties. How to analyse two different compounds to determine their differences in physical properties.
0:20 Intermolecular forces IMF
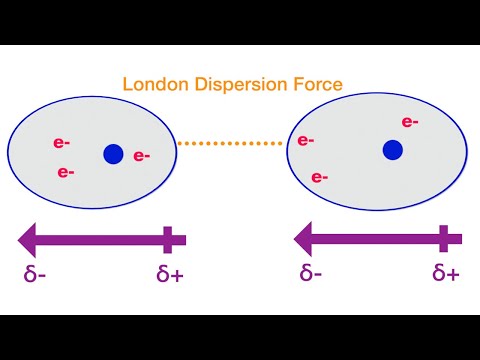
0:47 London dispersion forces
2:00 Dipole-dipole forces
2:36 Hydrogen bonding
3:26 IMF and physical properties
5:11 Example problems comparing molecules and IMF
4.4 Intermolecular forces SL
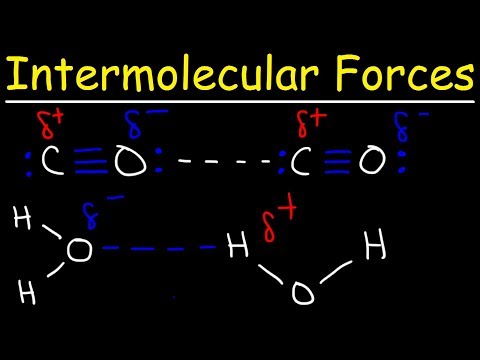
• Intermolecular forces include London dispersion forces, dipole-dipole forces and hydrogen bonding.
• The relative strengths of these interactions are London dispersion forces less than dipole-dipole forces less than hydrogen bonds.
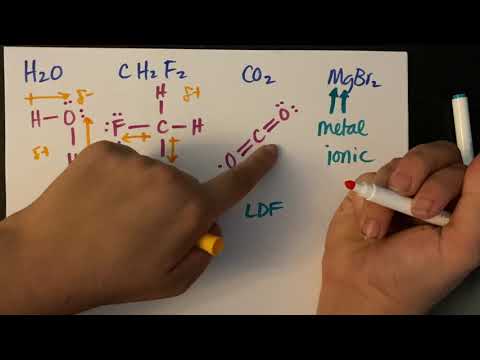
• Deduction of the types of intermolecular force present in substances, based on their structure and chemical formula.
• Explanation of the physical properties of covalent compounds - volatility, electrical conductivity and solubility, in terms of their structure and intermolecular forces.
Connect with me:
The different types of intermolecular forces - London dispersion forces, dipole-dipole forces, and hydrogen bonding. How the different intermolecular forces cause different physical properties. How to analyse two different compounds to determine their differences in physical properties.
0:20 Intermolecular forces IMF
0:47 London dispersion forces
2:00 Dipole-dipole forces
2:36 Hydrogen bonding
3:26 IMF and physical properties
5:11 Example problems comparing molecules and IMF
4.4 Intermolecular forces SL
• Intermolecular forces include London dispersion forces, dipole-dipole forces and hydrogen bonding.
• The relative strengths of these interactions are London dispersion forces less than dipole-dipole forces less than hydrogen bonds.
• Deduction of the types of intermolecular force present in substances, based on their structure and chemical formula.
• Explanation of the physical properties of covalent compounds - volatility, electrical conductivity and solubility, in terms of their structure and intermolecular forces.
Connect with me:
IB Chemistry Topic 4.4 Intermolecular forces
IB Chemistry Topic 4.4: Polarity, Intermolecular Forces & Solubility
Intermolecular Forces [IB Chemistry SL/HL]
Intermolecular Forces and Boiling Points
IB Chemistry Topic 4 - Notes D - Intermolecular Forces
Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubilit...
IB Chemistry: Intermolecular Forces
IB Chemistry SL Topic 4: Revision Lecture
VSEPR & Molecular Polarity [IB Chemistry SL/HL]
4.4/S2.2.8 What are Dipole-Dipole Intermolecular Forces? [SL IB Chemistry]
4.3.2 Describe & explain how intermolecular forces affect boiling points IB Chemistry SL
What Are Intermolecular Forces | Properties of Matter | Chemistry | FuseSchool
Cohesive thinking: Chemical bonding and intermolecular forces (Topic 4)
4.4.U.1 Intramolecular and Van Der Waals (Intermolecular) Forces - IB New Syllabus
4.4/S2.2.9 Deduction of intermolecular forces, via structure & chemical formula [SL IB Chemistry...
Intermolecular Forces 4: London Dispersion
London Dispersion Forces in 20 seconds
MATRICULATION CHEMISTRY SK015: 4.4 INTERMOLECULAR FORCES
IB 4.4 intermolecular forces & covalent properties
Topic 4: Intermolecular Forces 1(MATRICULATION)
Ranking Intermolecular Forces - Compare Highest/Lowest Boiling Points with IMF's
Solubility and intermolecular forces | AP Chemistry | Khan Academy
IB Chemistry 4.4: Intermolecular Forces - Hydrides of Groups 14 – 17
Reinforcement Exercise - 4.4 Intermolecular Forces [ Part 1]
Комментарии
 0:07:11
0:07:11
 0:04:24
0:04:24
 0:11:03
0:11:03
 0:10:54
0:10:54
 0:24:30
0:24:30
 0:10:40
0:10:40
 0:05:03
0:05:03
 0:53:21
0:53:21
 0:12:48
0:12:48
 0:07:55
0:07:55
 0:05:25
0:05:25
 0:05:19
0:05:19
 0:03:47
0:03:47
 0:11:46
0:11:46
 0:15:36
0:15:36
 0:01:43
0:01:43
 0:00:22
0:00:22
 0:18:11
0:18:11
 0:13:25
0:13:25
 0:09:05
0:09:05
 0:09:33
0:09:33
 0:04:23
0:04:23
 0:11:49
0:11:49
 0:07:05
0:07:05