filmov
tv
Heat Capacity

Показать описание
The heat capacity of an object describes how much heat is required to change its temperature. Because heat is a path function, the heat capacity at constant volume is different than the heat capacity at constant pressure.
Heat Capacity, Specific Heat, and Calorimetry
Heat capacity | Thermodynamics | AP Chemistry | Khan Academy
GCSE Physics - Internal Energy and Specific Heat Capacity #28
What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity
Specific Heat Capacity | Matter | Physics | FuseSchool
ADLC - Elementary Science: Heat Capacity
specific heat capacity explained
Heat Capacity
Heat, Specific Heat Capacity and Temperature // HSC Physics
What is Heat Capacity?
Calorimetry Examples: How to Find Heat and Specific Heat Capacity
What is the difference between Heat Capacity and Specific Heat Capacity?
Class 9 - Physics - Chapter 8 - Lecture 3 - 8.3 Specific Heat Capacity - Allied Schools
A sample specific heat capacity problem with solution
Calorimetry: Crash Course Chemistry #19
Solving Heat Capacity and Specific Heat Capacity problems - Pure Physics
Thermodynamics: Specific Heat Capacity Calculations
Concept of Heat Capacity | Heat
Specific Heat Capacity - GCSE Science Required Practical
Heat Capacity Introduction
Heat Capacity of Water
Heat capacity at constant volume and pressure | Physical Processes | MCAT | Khan Academy
Feel the Heat Capacity (In the Greenhouse #5)
Heat capacity at constant volume and pressure | Physics | Khan Academy
Комментарии
 0:04:14
0:04:14
 0:08:41
0:08:41
 0:04:36
0:04:36
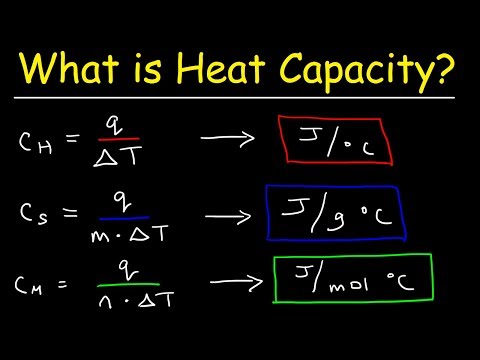 0:12:29
0:12:29
 0:03:14
0:03:14
 0:03:20
0:03:20
 0:09:50
0:09:50
 0:14:14
0:14:14
 0:09:33
0:09:33
 0:01:55
0:01:55
 0:04:13
0:04:13
 0:03:58
0:03:58
 0:17:27
0:17:27
 0:01:55
0:01:55
 0:11:57
0:11:57
 0:03:53
0:03:53
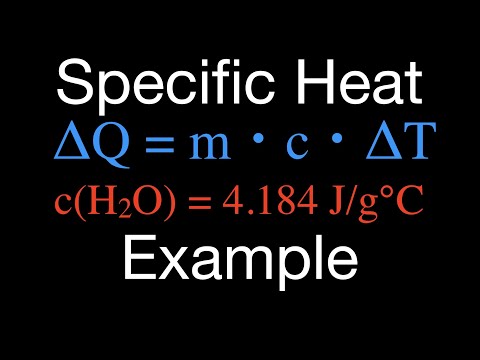 0:04:38
0:04:38
 0:04:54
0:04:54
 0:07:04
0:07:04
 0:04:04
0:04:04
 0:01:13
0:01:13
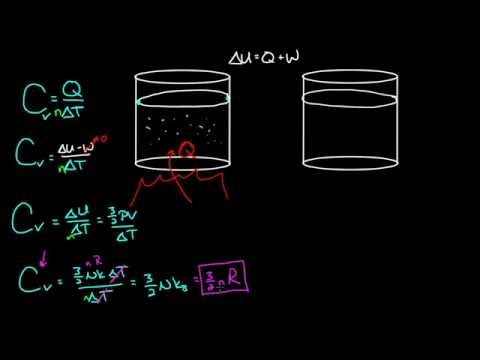 0:12:16
0:12:16
 0:03:23
0:03:23
 0:12:16
0:12:16