filmov
tv
specific heat capacity explained

Показать описание
This video covers specific heat capacity and uses the concept to explain why water is used as a coolant and explain why it coastal regions have differing temperature ranges than inland regions.
LIKE and SHARE with your peers. And please add a COMMENT to let me know I have helped you.
OR
Physics High is committed to producing content that teaches physics concepts at a level a high schooler can understand.
👥 Social
---------------------------------------------------------
Follow me on
facebook: @physicshigh
twitter: @physicshigh
Instagram: @physicshigh
#physicshigh #highschoolphysicsexplained
LIKE and SHARE with your peers. And please add a COMMENT to let me know I have helped you.
OR
Physics High is committed to producing content that teaches physics concepts at a level a high schooler can understand.
👥 Social
---------------------------------------------------------
Follow me on
facebook: @physicshigh
twitter: @physicshigh
Instagram: @physicshigh
#physicshigh #highschoolphysicsexplained
Heat Capacity, Specific Heat, and Calorimetry
specific heat capacity explained
Specific Heat Capacity | Matter | Physics | FuseSchool
GCSE Physics - Internal Energy and Specific Heat Capacity #28
What is the difference between Heat Capacity and Specific Heat Capacity?
Specific Heat Capacity Explained
Specific Heat Capacity - GCSE Science Required Practical
simple explanation of specific heat capacity & heat capacity and its relation with kinetic energ...
He Owns the Store That Transforms Ordinary People Into Legendary Heroes with a Single Purchase
Calorimetry: Crash Course Chemistry #19
Specific Heat Capacity Definition - A Level Physics
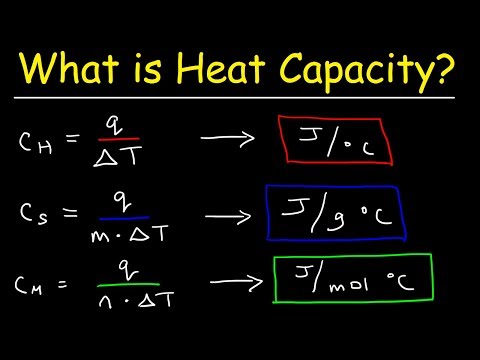
What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity
Specific Latent Heat | Matter | Physics | FuseSchool
Specific Heat Capacity - GCSE Physics
Specific Heat Capacity Definition - GCSE Physics
ADLC - Elementary Science: Heat Capacity
SPECIFIC HEAT - a quick definition
Specific heat of water | Water, acids, and bases | Biology | Khan Academy
Heat Capacity and Specific Heat
Class 11th – Specific Heat Capacity | Thermal Properties of Matter | Tutorials Point
Specific Heat Capacity | Heat
GCSE Physics - Specific Latent Heat #29
Specific Heat Capacity 2 - GCSE Physics Worksheet Answers EXPLAINED
Thermodynamics SPECIFIC HEATS - cv & cp - in 12 Minutes!
Комментарии
 0:04:14
0:04:14
 0:09:50
0:09:50
 0:03:14
0:03:14
 0:04:36
0:04:36
 0:03:58
0:03:58
 0:13:20
0:13:20
 0:07:04
0:07:04
 0:15:39
0:15:39
 11:05:53
11:05:53
 0:11:57
0:11:57
 0:00:09
0:00:09
 0:12:29
0:12:29
 0:03:55
0:03:55
 0:04:48
0:04:48
 0:00:09
0:00:09
 0:03:20
0:03:20
 0:01:05
0:01:05
 0:10:49
0:10:49
 0:05:21
0:05:21
 0:22:21
0:22:21
 0:12:45
0:12:45
 0:06:26
0:06:26
 0:04:07
0:04:07
 0:12:39
0:12:39