filmov
tv
Solving Heat Capacity and Specific Heat Capacity problems - Pure Physics
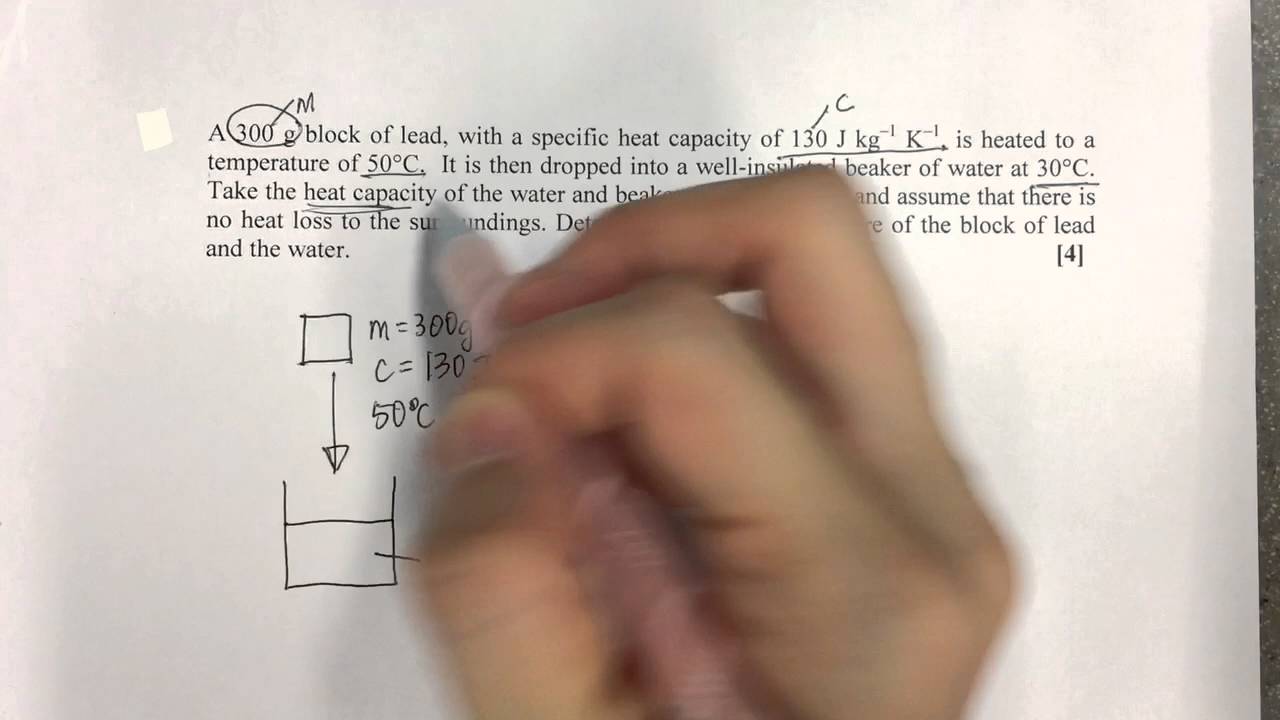
Показать описание
Calorimetry Examples: How to Find Heat and Specific Heat Capacity
Solving Heat Capacity and Specific Heat Capacity problems - Pure Physics
Heat Capacity, Specific Heat, and Calorimetry
What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity
Specific Heat Capacity - Solving for Change in Temperature
Specific Heat Capacity - Solving for Joules
How To Solve Basic Calorimetry Problems in Chemistry
specific heat capacity explained
Capacitor Energy Loss Explained! 🔋@PFNEET#physicsforneet #neetstudytips #neet2025physics
Calculations involving heat and specific heat
Heat Capacity and Specific Heat - Chemistry Tutorial
GCSE Physics - Internal Energy and Specific Heat Capacity #28
Specific Heat Capacity - Solving for Initial Temperature
A sample specific heat capacity problem with solution
Final Temperature Calorimetry Practice Problems - Chemistry
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial and Final Temperature, Mass
Using the formula q=mcΔT (Three examples)
Specific Heat Capacity | Matter | Physics | FuseSchool
Heat capacity | Thermodynamics | AP Chemistry | Khan Academy
Specific Heat Capacity and Latent Heat Calculations
Specific Heat Capacity Calculations. Easy to Hard. E=mcT
Thermodynamics: Specific Heat Capacity Calculations
Heat capacity at constant volume and pressure | Physical Processes | MCAT | Khan Academy
Heat Capacity and Specific Heat
Комментарии
 0:04:13
0:04:13
 0:03:53
0:03:53
 0:04:14
0:04:14
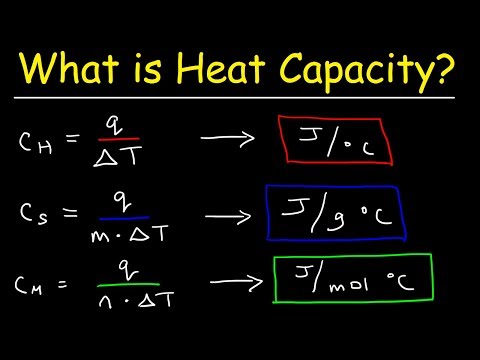 0:12:29
0:12:29
 0:01:43
0:01:43
 0:02:16
0:02:16
 0:10:25
0:10:25
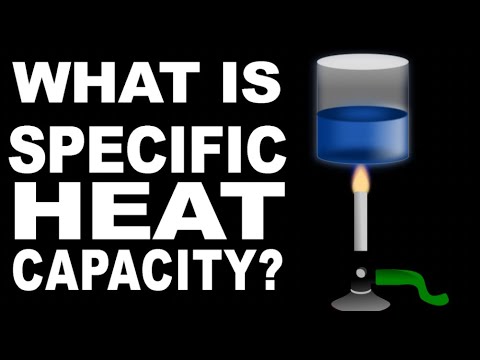 0:09:50
0:09:50
 0:00:56
0:00:56
 0:05:33
0:05:33
 0:06:05
0:06:05
 0:04:36
0:04:36
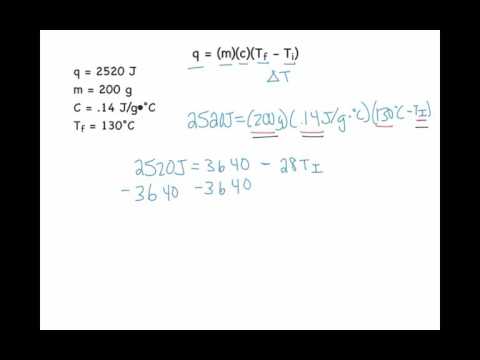 0:04:28
0:04:28
 0:01:55
0:01:55
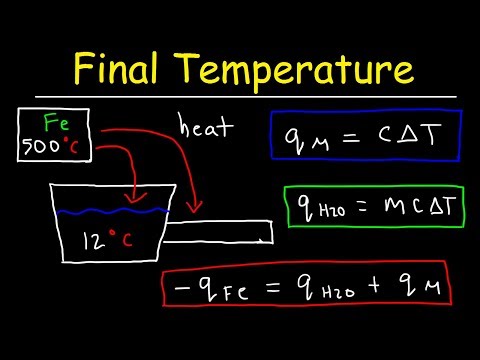 0:18:16
0:18:16
 0:09:19
0:09:19
 0:07:01
0:07:01
 0:03:14
0:03:14
 0:08:41
0:08:41
 0:03:46
0:03:46
 0:08:51
0:08:51
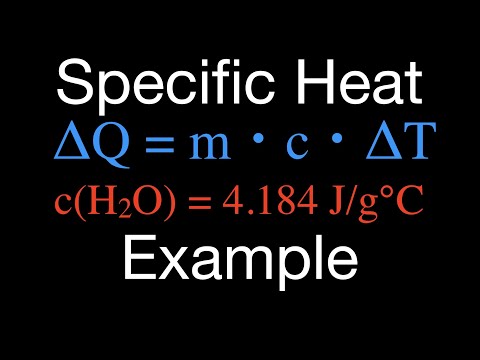 0:04:38
0:04:38
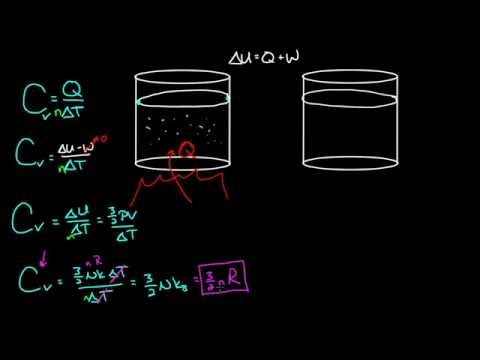 0:12:16
0:12:16
 0:05:21
0:05:21