filmov
tv
Delta G, Delta H, and Delta S Problem (AP Chemistry)

Показать описание
Delta G (Gibbs Free Energy), Delta H (Enthalpy), and Delta S (Entropy) define whether a reaction will be thermodynamically favorable or thermodynamically unfavorable. These terms are often also referred to as "spontaneous" or "non-spontaneous." This is a multiple choice question (MCQ) that is specifically designed to prepare for the AP Chemistry test, but may also be relevant for other exams and curricula such as A-levels, IGCSE, JEE, NEET, MCAT, DAT, OAT, GRE and SAT.
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Delta G, Delta H, and Delta S Problem (AP Chemistry)
18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemis...
18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, and Delta S
Using Gibbs Free Energy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
18.4 Calculating Delta G, Delta H, & Delta S | General Chemistry
Introduction to Gibbs free energy | Applications of thermodynamics | AP Chemistry | Khan Academy
Sporting Delta 1 - HKV Ons Eibernest 1
18 Thermodynamics -- Delta G, Delta H, and Delta S
7: Using enthalpy and entropy to predict delta G
18.5 Gibbs Free Energy and the Equilibrium Constant
Gibbs Free Energy
Temperature Dependence and Delta G
R1.4.2 Explain how Temperature Changes Delta G [HL IB Chemistry]
What is delta G?
AP Chemistry Notes 9.3- Delta G, H, and S
How to Calculate Delta G using Delta H and Delta S
Relationship Between Delta G and the Equilibrium Constant
Canu delta G = delta H Temp times Delta S
18.4 Delta G, Delta H, Delta S and Formation Reactions
6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry
CH16Q8 Finding delta G from delta H and delta S Lower Temp
How to Calculate ∆G° Standard Gibb's Free Energy Change of a Reaction from Enthalpy H and Entro...
Комментарии
 0:08:12
0:08:12
 0:04:50
0:04:50
 0:32:10
0:32:10
 0:22:42
0:22:42
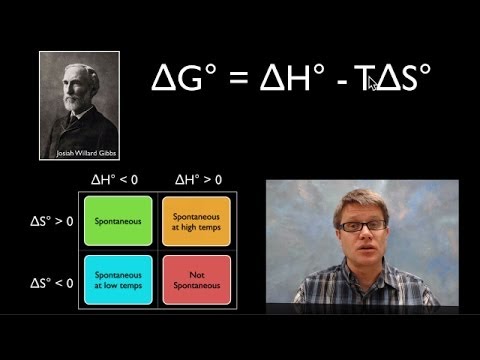 0:07:57
0:07:57
 0:44:45
0:44:45
 0:18:12
0:18:12
 0:05:39
0:05:39
 1:37:25
1:37:25
 1:07:00
1:07:00
 0:08:02
0:08:02
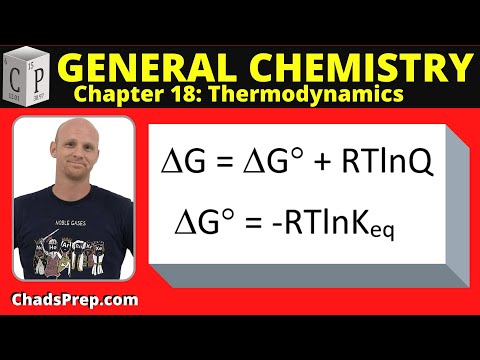 0:11:12
0:11:12
 0:14:13
0:14:13
 0:06:22
0:06:22
 0:04:57
0:04:57
 0:05:56
0:05:56
 0:10:17
0:10:17
 0:02:30
0:02:30
 0:11:46
0:11:46
 0:07:38
0:07:38
 0:14:33
0:14:33
 0:17:55
0:17:55
 0:01:51
0:01:51
 0:04:05
0:04:05