filmov
tv
15. Thermodynamics: Bond and Reaction Enthalpies

Показать описание
MIT 5.111 Principles of Chemical Science, Fall 2014
Instructor: Catherine Drennan
Thermodynamics is key to understanding the reactivity of molecules and compounds. In this first of three lectures on thermodynamics, viewers are introduced to ∆H, and asked to consider how much heat it will take to break one type of molecular bond versus another. Viewers are also asked whether a particular chemical reaction will release heat or absorb heat.
License: Creative Commons BY-NC-SA
Instructor: Catherine Drennan
Thermodynamics is key to understanding the reactivity of molecules and compounds. In this first of three lectures on thermodynamics, viewers are introduced to ∆H, and asked to consider how much heat it will take to break one type of molecular bond versus another. Viewers are also asked whether a particular chemical reaction will release heat or absorb heat.
License: Creative Commons BY-NC-SA
15. Thermodynamics: Bond and Reaction Enthalpies
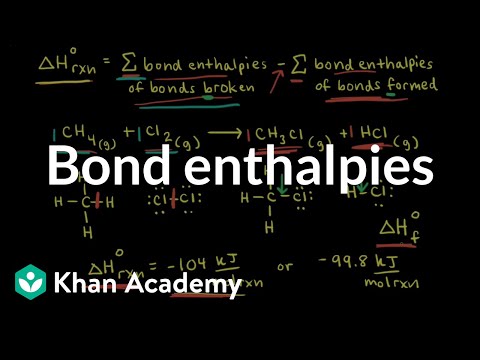
Bond enthalpies | Thermodynamics | AP Chemistry | Khan Academy
Thermodynamics and Energy Diagrams: Crash Course Organic Chemistry #15
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Kinetic vs Thermodynamic Product - 1,2 vs 1,4 Addition of HBr to 1,3- Butadiene
Enthalpy: Crash Course Chemistry #18
Tricks to solve Thermochemistry problems easily | Enthalpy of formation combustion
Plus One Chemistry - Thermodynamics - Numerical Problems | Xylem Plus One
First day of the Finals of the Dream Chemistry Award 2024
Enthalpy of Reaction
Bro’s hacking life 😭🤣
Top 10 Tricks from Thermodynamics & Thermochemistry
Enthalpy of Reaction from Bond Energy | Hess Law | Thermodynamics | Chemistry |
IIT Bombay Lecture Hall | IIT Bombay Motivation | #shorts #ytshorts #iit
Introduction to Thermodynamics of Reactions (Q&A)
Enthalpy of Reaction - Thermodynamics (Part 15)
Enthalpy of formation | Thermodynamics | AP Chemistry | Khan Academy
Akansha Ma’am at Unacademy Neet Centre
Is??? Alakh sir is only 12th pass || #physicswallah #ashortaday
Isomers Explained #chemistry #organicchemistry #shorts
One shot | 4 chemical thermodynamics chemistry class 12 MAHARASHTRA BOARD with handwritten notes
Thermodynamics (Bond dissociation energy) part 1
How to Balance Chemical Equations
Anushka Mam got angry on student #pwians #physicswallah #shorts #ytshortsindia
Комментарии
 0:38:21
0:38:21
 0:09:33
0:09:33
 0:11:12
0:11:12
 0:08:12
0:08:12
 0:12:51
0:12:51
 0:11:24
0:11:24
 0:17:40
0:17:40
 0:22:55
0:22:55
 3:08:10
3:08:10
 0:08:03
0:08:03
 0:00:20
0:00:20
 0:22:05
0:22:05
 0:09:53
0:09:53
 0:00:12
0:00:12
 0:41:08
0:41:08
 0:17:28
0:17:28
 0:09:25
0:09:25
 0:00:16
0:00:16
 0:00:52
0:00:52
 0:00:22
0:00:22
 0:57:31
0:57:31
 0:17:12
0:17:12
 0:02:25
0:02:25
 0:00:53
0:00:53