filmov
tv
Factors that affect Rate of Reaction

Показать описание
What are the four factors that affect the rate of reaction within a chemical reaction?
There are four factors that impact the rate of a reaction.
Temperature, Concentration, Surface area, Inhibitors, and Catalysts.
0:00 Introduction Rates of Reaction
0:28 Activation Energy
0:52 Factors that impact the rate of reaction
1:09 Temperature
1:27 Concentration
1:49 Surface Area
2:12 Inhibitors Corrosive, Enzyme, Rection
2:40 Catalyst
Transcript
There are four factors that impact the rate of a reaction.
Temperature, Concentration, Surface area, Inhibitors, and Catalysts.
0:00 Introduction Rates of Reaction
0:28 Activation Energy
0:52 Factors that impact the rate of reaction
1:09 Temperature
1:27 Concentration
1:49 Surface Area
2:12 Inhibitors Corrosive, Enzyme, Rection
2:40 Catalyst
Transcript
GCSE Chemistry - Factors Affecting the Rate of Reaction #47
Factors that affect Rate of Reaction
Rate of Dissolving and Factors that Affect It
Factors That Affect the Rate of Photosynthesis | Biology for All | FuseSchool
How to speed up chemical reactions (and get a date) - Aaron Sams
Rates of Reaction - Part 2 | Reactions | Chemistry | FuseSchool
GCSE Biology - Enzymes - How Temperature and pH Affect Rate of Reaction
Factors that Affect Reaction Rate
Housing Market On Verge of Collapse: 56,000+ Homes Cancelled.
R2.2.3 Factors that affect the rate of reaction
6.1 Factors that affect the rate of reaction (SL)
Factors which Affect the Rate of Chemical Reactions
What is Diffusion? How Does it Work? What Factors Affect it? #7
application of factors that affect rate of reaction in daily life
Investigating The Limiting Factors That Affect The Rate Of Photosynthesis | IGCSE and GCSE Biology
GCSE Chemistry Factors Which Affect Rate of Reactions Revision
Factors That Affect The Reaction Rate (Temperature)
Rates of Reactions - Part 1 | Reactions | Chemistry | FuseSchool
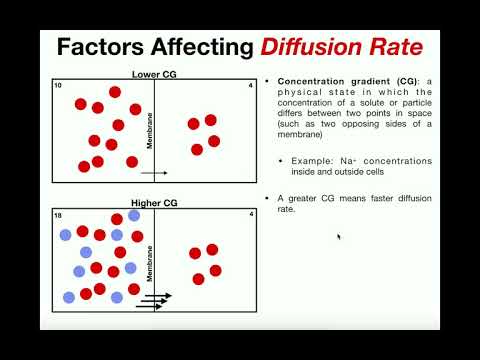
Factors that Affect Diffusion Rate
Factors that Affect the Rate of Dissolving
Rate of Reactions 3 — Factors that affect Rate
Chemistry 11.4 Factors that affect Reaction Rate
Factors that affect rate of reaction and particle theory
How does concentration affect reaction rate?
Комментарии
 0:05:15
0:05:15
 0:03:07
0:03:07
 0:10:18
0:10:18
 0:03:04
0:03:04
 0:04:56
0:04:56
 0:05:41
0:05:41
 0:03:37
0:03:37
 0:14:00
0:14:00
 1:14:33
1:14:33
 0:04:41
0:04:41
 0:03:05
0:03:05
 0:01:44
0:01:44
 0:05:18
0:05:18
 0:01:22
0:01:22
 0:07:14
0:07:14
 0:05:55
0:05:55
 0:02:00
0:02:00
 0:04:27
0:04:27
 0:17:03
0:17:03
 0:02:09
0:02:09
 0:09:43
0:09:43
 0:10:39
0:10:39
 0:04:24
0:04:24
 0:01:11
0:01:11