filmov
tv
Calculate the standard Gibbs free energy change from the free energies of formation data

Показать описание
Calculate the standard Gibbs free energy change from the free energies of formation data for the following reaction: `C_(6)H_(6)(l) +(15)/(2)O_(2)(g) rarr 6CO_(2)(g) +3H_(2)O(g)` Given that `Delta_(f)G^(Theta) =[C_(6)H_(6)(l)] = 172.8 kJ mol^(-1)` `Delta_(f)G^(Theta)[CO_(2)(g)] =- 394.4 kJ mol^(-1)` `Delta_(f)G^(Theta) [H_(2)O(g)] =- 228.6 kJ mol^(-1)`
 0:44:45
0:44:45
 0:05:25
0:05:25
 0:05:39
0:05:39
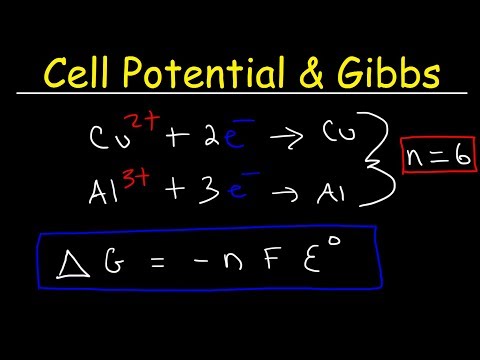 0:11:02
0:11:02
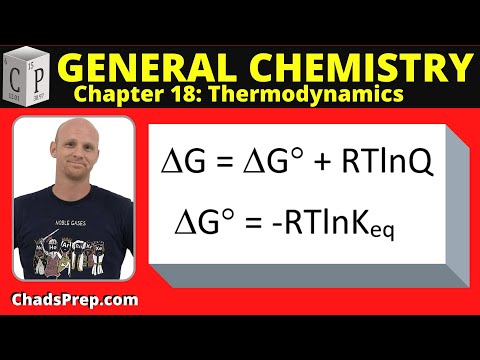 0:11:12
0:11:12
 0:04:05
0:04:05
 0:05:12
0:05:12
 0:03:29
0:03:29
 0:24:43
0:24:43
 0:06:01
0:06:01
 0:04:28
0:04:28
 0:04:57
0:04:57
 0:03:02
0:03:02
 0:06:05
0:06:05
 0:10:53
0:10:53
 0:04:26
0:04:26
 0:05:26
0:05:26
 0:01:58
0:01:58
 0:05:22
0:05:22
 0:05:33
0:05:33
 0:03:48
0:03:48
 0:06:04
0:06:04
 0:03:19
0:03:19
 0:01:59
0:01:59