filmov
tv
E1 Reaction

Показать описание
Here we examine the mechanism for the E1 reaction. Don't worry, it's just these four for now!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
E1 Reaction Mechanism With Alcohol Dehydration & Ring Expansion Problems
E1 Reaction
E1 Reaction Rate and Mechanism - Unimolecular beta-elimination (vid 1 of 3) by Leah4sci
E1 and E2 Elimintaion Reactions | Mechanism
E1 and E2 Reactions: Crash Course Organic Chemistry #22
E1 reactions | Substitution and elimination reactions | Organic chemistry | Khan Academy
7.6 E1 Reactions and E1 vs E2 | Organic Chemistry
SN2 SN1 E1 E2 Reaction Mechanisms Made Easy!
Organic Chemistry 1 - Questions of SN1 and E1 reactions
E1 mechanism: carbocations and rearrangements
E1 Reactions
SN1 SN2 E1 E2 Reaction Mechanism Overview
Choosing Between SN2, SN1, E2 and E1 Reactions
Determining SN1, SN2, E1, and E2 Reactions: Crash Course Organic Chemistry #23
Choosing Between SN1/SN2/E1/E2 Mechanisms
E1 mechanism with examples
E2 and E1 Elimination Made Easy! Part 1 ( Mechanisms and Beta Hydrogens ) - Organic Chemistry
Choosing Between SN1 SN2 E1 E2 Reactions
Predict the Major Product in This E1 Reaction
7 E1 vs E2
Elimination E1 : Mécanisme
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement
E2 Reaction Mechanism - Hoffman Elimination vs Zaitsev's Rule
E1 vs E2 reactions Trick | Class 12 | IIT JEE | NEET | Vineet Khatri | ATP STAR
Комментарии
 0:12:37
0:12:37
 0:02:03
0:02:03
 0:09:00
0:09:00
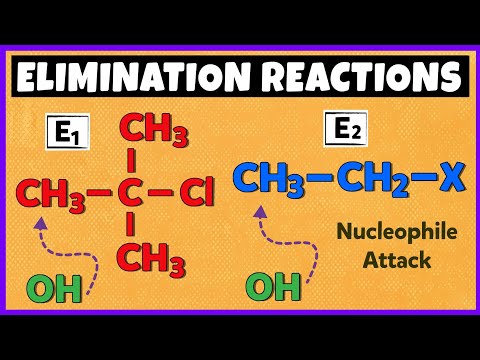 0:16:06
0:16:06
 0:13:58
0:13:58
 0:09:22
0:09:22
 0:15:26
0:15:26
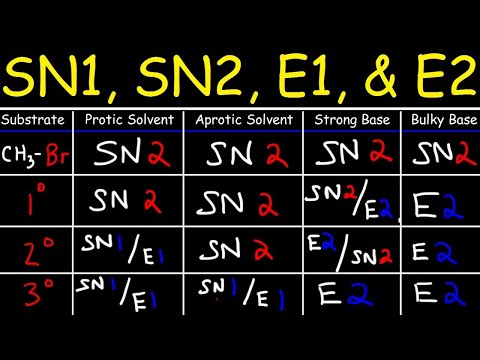 0:38:50
0:38:50
 0:26:25
0:26:25
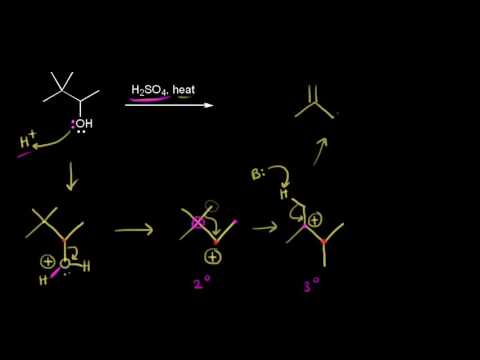 0:10:29
0:10:29
 0:10:58
0:10:58
 0:07:29
0:07:29
 0:09:06
0:09:06
 0:13:31
0:13:31
 0:18:52
0:18:52
 0:14:17
0:14:17
 0:06:15
0:06:15
 0:12:31
0:12:31
 0:06:14
0:06:14
 0:46:33
0:46:33
 0:09:50
0:09:50
 0:34:45
0:34:45
 0:12:20
0:12:20
 0:09:51
0:09:51