filmov
tv
E1 Reaction Mechanism With Alcohol Dehydration & Ring Expansion Problems
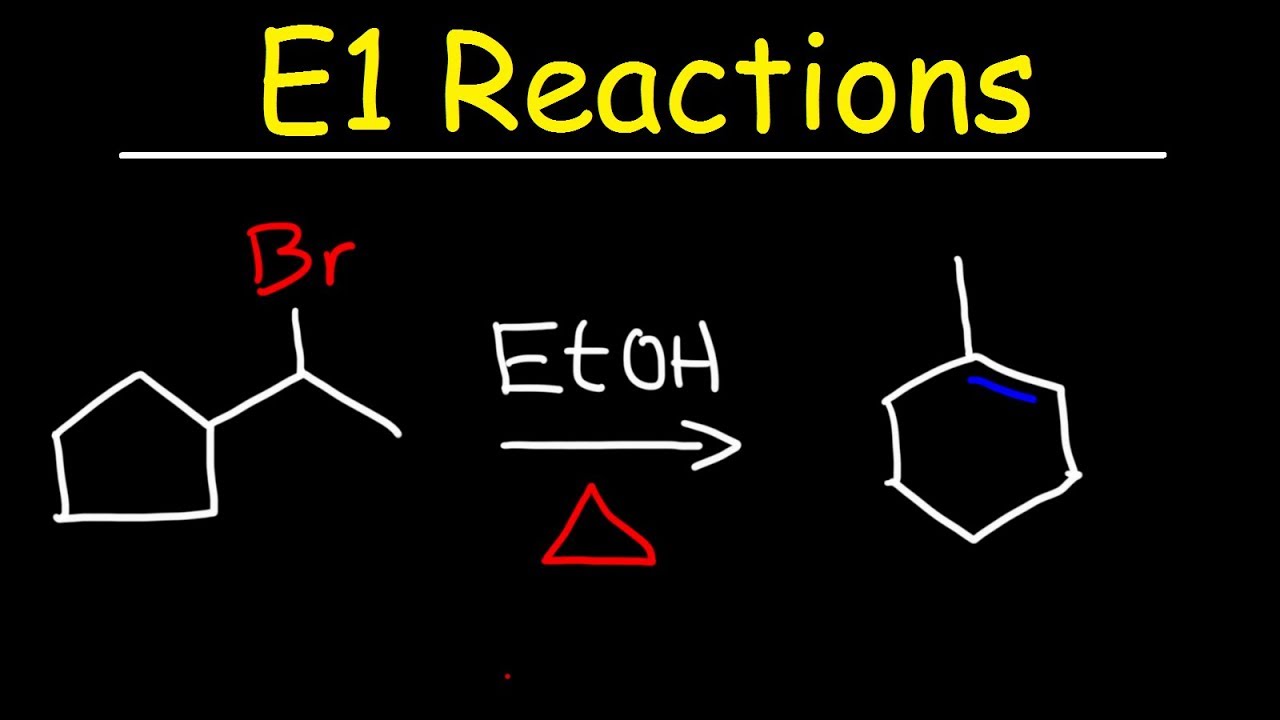
Показать описание
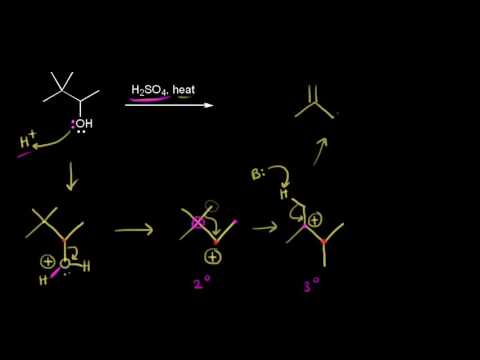
This organic chemistry video tutorial provides a basic introduction into the E1 reaction mechanism. It includes example problems with carbocation rearrangements such as the hydride shift, methyl shift, and the ring expansion. It discusses E1 elimination reactions of alkyl halides and the E1 dehydration reaction of alcohols using H2SO4.
Stereochemistry R/S Configuration:
Optical Activity & Specific Rotation:
SN1, SN2, E1, E2 Reaction Mechanisms:
SN2 Reaction Mechanisms:
SN2 - Test Question:
_______________________________
SN1 Reaction Mechanisms:
Carbocation Stability - Hyperconjugation:
Carbanion Stability:
Protic Vs Aprotic Solvents:
E1 Ring Expansion:
E2 - Test Question:
________________________________
E2 Stereochemistry - Newman Projections:
SN1, SN2, E1, E2 - Practice Test:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Full-Length Videos and Worksheets:
Stereochemistry R/S Configuration:
Optical Activity & Specific Rotation:
SN1, SN2, E1, E2 Reaction Mechanisms:
SN2 Reaction Mechanisms:
SN2 - Test Question:
_______________________________
SN1 Reaction Mechanisms:
Carbocation Stability - Hyperconjugation:
Carbanion Stability:
Protic Vs Aprotic Solvents:
E1 Ring Expansion:
E2 - Test Question:
________________________________
E2 Stereochemistry - Newman Projections:
SN1, SN2, E1, E2 - Practice Test:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Full-Length Videos and Worksheets:
Комментарии
 0:12:37
0:12:37
 0:02:03
0:02:03
 0:11:18
0:11:18
 0:16:59
0:16:59
 0:03:16
0:03:16
 0:13:58
0:13:58
 0:10:58
0:10:58
 0:09:22
0:09:22
 0:16:06
0:16:06
 0:18:56
0:18:56
 0:38:50
0:38:50
 0:05:22
0:05:22
 0:34:45
0:34:45
 0:04:33
0:04:33
 0:01:53
0:01:53
 0:12:01
0:12:01
 0:04:17
0:04:17
 0:15:26
0:15:26
 0:06:50
0:06:50
 0:09:49
0:09:49
 0:10:29
0:10:29
 0:14:17
0:14:17
 0:04:54
0:04:54
 0:59:10
0:59:10