filmov
tv
9.1/S3.1.6 How do I Assign Oxidation States? [SL IB Chemistry]
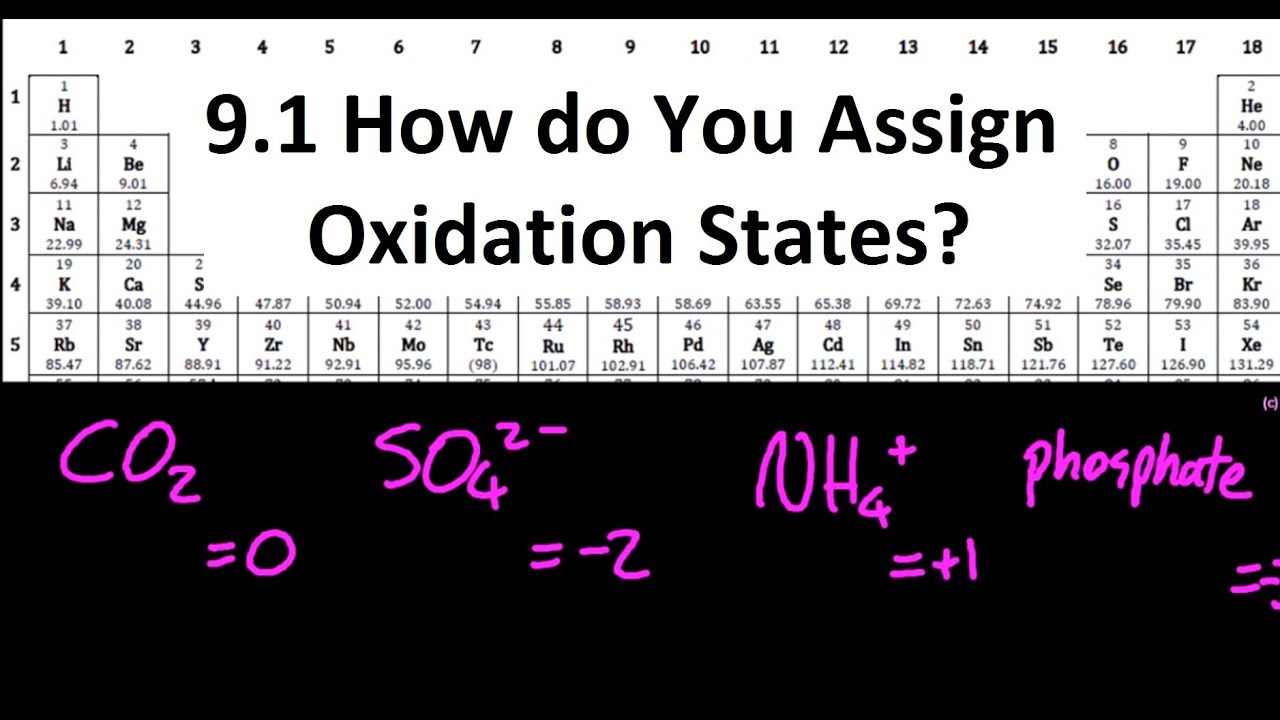
Показать описание
1) OS of elements are zero
2) Sum of OS = charge on the molecule/ion
3) OS of H in a compound is +1 (except when with a metal OS=-1)
4) OS of O in a compound is -2 (except in H2O2 where OS = -1)
5) Fluorine in a compound is -1
6) OS of metal in a compound = +(valence electrons)
7) Halogen = meh probably -1.
2) Sum of OS = charge on the molecule/ion
3) OS of H in a compound is +1 (except when with a metal OS=-1)
4) OS of O in a compound is -2 (except in H2O2 where OS = -1)
5) Fluorine in a compound is -1
6) OS of metal in a compound = +(valence electrons)
7) Halogen = meh probably -1.
9.1/S3.1.6 How do I Assign Oxidation States? [SL IB Chemistry]
Huffman coding || Easy method
IP|3 How to add IP camera - Dahua
3. Infix to Postfix Conversion The Easy Way
Miller indices simplest explaination| animation
AWS S3 Tutorial For Beginners
This is Why You Never Mess With a Royal Guard...
Lec-29 Assignment Problem Hungarian Method | In Hindi | Operation Research
Representation of signed number | sign magnitude form | 1's complement and 2's complement...
Spektrum AS3X Step 1: Transmitter Setup (Initial Setup)
How to Program your Android TV box Remote
Pro Tools Quick Tips: I/O Setup - Inputs and Outputs
Integration of H NMR Signals - Spectroscopy - Organic Chemistry
Least cost method[transportation problem] in operation research.
A Surprise Guest for Shaun and Lea - The Good Doctor
No 6: FOOT SWITCH ASSIGN to the SQ Mixer
Evaluation of Postfix Expression | Examples | Data Structures | Lec-20 | Bhanu Priya
ONTAP 9.10.1 - UX - Case 10 - Assigning QoS to LUNs instead of parent volume
Tuya Smart Door Lock Fingerprint and Passcode Setup
The Platform | Main Trailer | Netflix
Boy Adopted by Cop Who Saved Him From Murderous Dad
The Crayon Song - Studio C
Can you solve the locker riddle? - Lisa Winer
Assigning formulae to mass spectral peaks by accurate mass
Комментарии
 0:08:43
0:08:43
 0:04:36
0:04:36
 0:00:40
0:00:40
 0:05:44
0:05:44
 0:05:13
0:05:13
 0:27:18
0:27:18
 0:08:13
0:08:13
 0:23:30
0:23:30
 0:05:56
0:05:56
 0:02:17
0:02:17
 0:01:51
0:01:51
 0:01:17
0:01:17
 0:05:29
0:05:29
 0:11:08
0:11:08
 0:01:29
0:01:29
 0:02:24
0:02:24
 0:04:23
0:04:23
 0:00:48
0:00:48
 0:05:46
0:05:46
 0:01:59
0:01:59
 0:02:29
0:02:29
 0:03:24
0:03:24
 0:03:50
0:03:50
 0:48:47
0:48:47