filmov
tv
Integration of H NMR Signals - Spectroscopy - Organic Chemistry
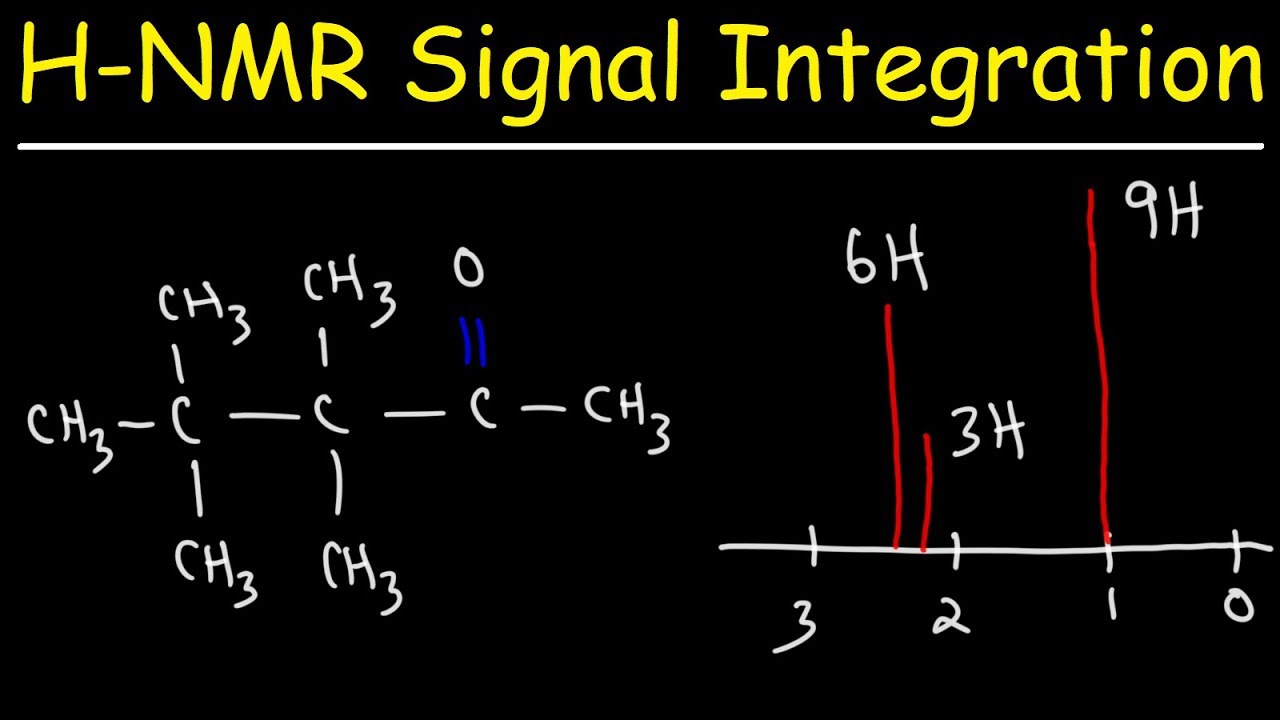
Показать описание
This organic chemistry video discusses the integration of H-NMR signals in NMR spectroscopy. It relates the area under the curve with the height of the integral trace to the relative number of protons in the molecule.
Alcohol Reactions - HBr, PBr3, & SOCl2:
Free Radical Reactions:
Reactions Summary:
Organic Chemistry 1 Final Exam Review:
IR Spectroscopy:
Mass Spectrometry:
______________________________
Proton NMR Spectroscopy:
Carbon-13 NMR Spectroscopy:
Ethers and Epoxides:
Diels Alder Reaction:
Organic Chemistry 2 Final Exam Review:
Alcohol Reactions - HBr, PBr3, & SOCl2:
Free Radical Reactions:
Reactions Summary:
Organic Chemistry 1 Final Exam Review:
IR Spectroscopy:
Mass Spectrometry:
______________________________
Proton NMR Spectroscopy:
Carbon-13 NMR Spectroscopy:
Ethers and Epoxides:
Diels Alder Reaction:
Organic Chemistry 2 Final Exam Review:
Integration of H NMR Signals - Spectroscopy - Organic Chemistry
peak integration in 1H NMR spectroscopy
Intensity of 1H NMR signals (Integral value)
15.5b The Integration or Area Under a Signal in Proton NMR | Organic Chemistry
How To Determine The Number of Signals In a H NMR Spectrum
NMR Spectroscopy Part 6 - Integration of NMR signals
NMR 5 - Integration
Proton NMR Integration Ratio
H-NMR Predicting Molecular Structure Using Formula + Graph
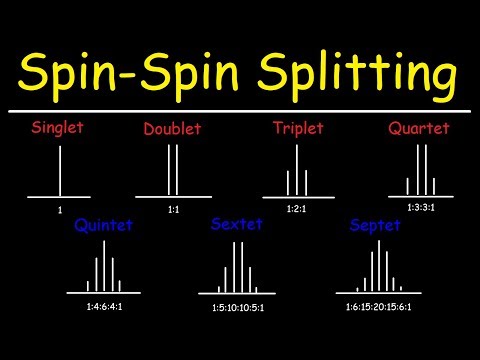
Spin Spin Splitting - N+1 Rule - Multiplicity - Proton NMR Spectroscopy
How To Draw The Proton NMR Spectrum of an Organic Molecule
Number of 1H NMR signals
NMR-Integration
NMR Spectroscopy
NMR Spectroscopy: Number of Signals and Peak Integration
1H NMR - Spectra Interpretation Part I Examples
NMR - 5. Integration
Proton NMR Spectroscopy - How To Draw The Structure Given The Spectrum
Ch15.21 - Integration in 1H NMR
Integration | Spectroscopy | Organic chemistry | Khan Academy
H-NMR Example Matching The Molecule To The Graph
15.3 The Number of Signals in Proton NMR | Organic Chemistry
Topspin 3.5.7 NMR - Integrating the peaks
15.5c The Splitting or Multiplicity in Proton NMR | Organic Chemistry
Комментарии
 0:05:29
0:05:29
 0:03:34
0:03:34
 0:04:49
0:04:49
 0:01:47
0:01:47
 0:20:26
0:20:26
 0:03:20
0:03:20
 0:17:24
0:17:24
 0:04:03
0:04:03
 0:11:02
0:11:02
 0:22:02
0:22:02
 0:12:06
0:12:06
 0:00:56
0:00:56
 0:02:51
0:02:51
 0:14:36
0:14:36
 0:22:20
0:22:20
 0:10:19
0:10:19
 0:14:30
0:14:30
 0:14:12
0:14:12
 0:03:43
0:03:43
 0:04:30
0:04:30
 0:06:43
0:06:43
 0:10:38
0:10:38
 0:07:59
0:07:59
 0:04:55
0:04:55