filmov
tv
Molarity - Chemistry Tutorial

Показать описание
This tutorial is designed to illustrate the concept of molarity and includes several examples of how to calculate molarity and to use molarity values in calculations.
Molarity - Chemistry Tutorial
Molarity Practice Problems
Molarity Made Easy: How to Calculate Molarity and Make Solutions
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
Molarity and Dilution
How to Do Solution Stoichiometry Using Molarity as a Conversion Factor | How to Pass Chemistry
Trick to Calculate Molarity | Molarity practice problems
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
Polytechnic 1st Semester Applied Chemistry| Chemical Bonding Class-02||By Harshit Pandey
Molarity | Intermolecular forces and properties | AP Chemistry | Khan Academy
Concentration and Molarity explained: what is it, how is it used + practice problems
Ion Concentration in Solutions From Molarity, Chemistry Practice Problems
Molarity Calculations Tutorial
Molarity - Chemistry Tutorial
4.4 Molarity and Dilutions | General Chemistry
Solution Stoichiometry - Finding Molarity, Mass & Volume
Solution Stoichiometry tutorial: How to use Molarity + problems explained | Crash Chemistry Academy
molarity tutorial
Molarity Mini Lesson
Introduction to Moles
Molarity Molality and Molar Mass for MCAT General Chemistry
An Actually Good Explanation of Moles
Molarity Tutorial
GCSE Chemistry - The Mole (Higher Tier) #25
Комментарии
 0:04:54
0:04:54
 0:21:27
0:21:27
 0:08:46
0:08:46
 0:31:25
0:31:25
 0:02:30
0:02:30
 0:07:38
0:07:38
 0:09:36
0:09:36
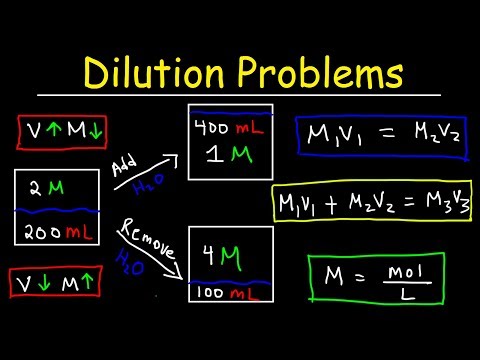 0:21:55
0:21:55
 0:39:10
0:39:10
 0:05:03
0:05:03
 0:05:41
0:05:41
 0:12:24
0:12:24
 0:04:56
0:04:56
 0:04:54
0:04:54
 0:16:12
0:16:12
 0:23:11
0:23:11
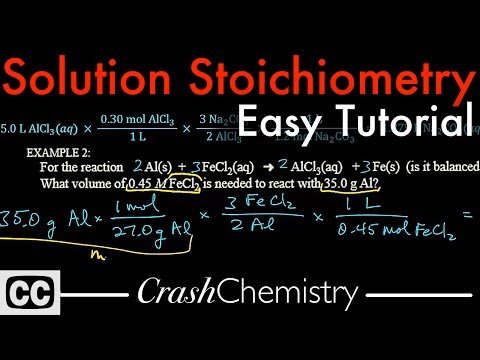 0:10:56
0:10:56
 0:17:23
0:17:23
 0:04:09
0:04:09
 0:05:16
0:05:16
 0:21:50
0:21:50
 0:13:37
0:13:37
 0:08:15
0:08:15
 0:04:29
0:04:29