filmov
tv
Solution Stoichiometry tutorial: How to use Molarity + problems explained | Crash Chemistry Academy

Показать описание
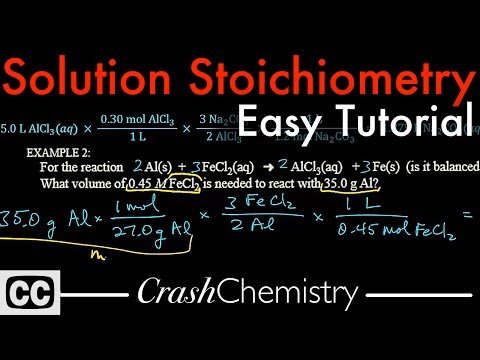
A tutorial on aqueous solutions and molarity, and then a detailed explanation of how to set up calculations for five example problems of solution stoichiometry involving molarity -- how to use molarity in stoichiometric conversions. Also includes problems using density and mass conversions!
CC Academy videos are crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
—More on Stoichiometry | Wikipedia—
"Stoichiometry...is the calculation of relative quantities of reactants and products in chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated....
Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.
Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.
Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio."
Wikipedia contributors. "Stoichiometry." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 27 May. 2016. Web. 27 May. 2016.
CC Academy videos are crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
—More on Stoichiometry | Wikipedia—
"Stoichiometry...is the calculation of relative quantities of reactants and products in chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated....
Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.
Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.
Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio."
Wikipedia contributors. "Stoichiometry." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 27 May. 2016. Web. 27 May. 2016.
Комментарии
 0:10:56
0:10:56
 0:15:24
0:15:24
 0:23:11
0:23:11
 0:07:09
0:07:09
 0:07:38
0:07:38
 0:25:16
0:25:16
 0:07:30
0:07:30
 0:12:40
0:12:40
 0:26:01
0:26:01
 0:09:33
0:09:33
 0:19:19
0:19:19
 0:11:34
0:11:34
 0:13:30
0:13:30
![Solution Stoichiometry [archived]](https://i.ytimg.com/vi/cXvOzICQrmo/hqdefault.jpg) 0:07:54
0:07:54
 0:09:51
0:09:51
 0:30:43
0:30:43
 0:06:47
0:06:47
 0:07:16
0:07:16
 0:08:10
0:08:10
 0:08:42
0:08:42
 0:03:36
0:03:36
 0:12:04
0:12:04
 0:08:43
0:08:43
 0:00:58
0:00:58