filmov
tv
Molarity and Dilution
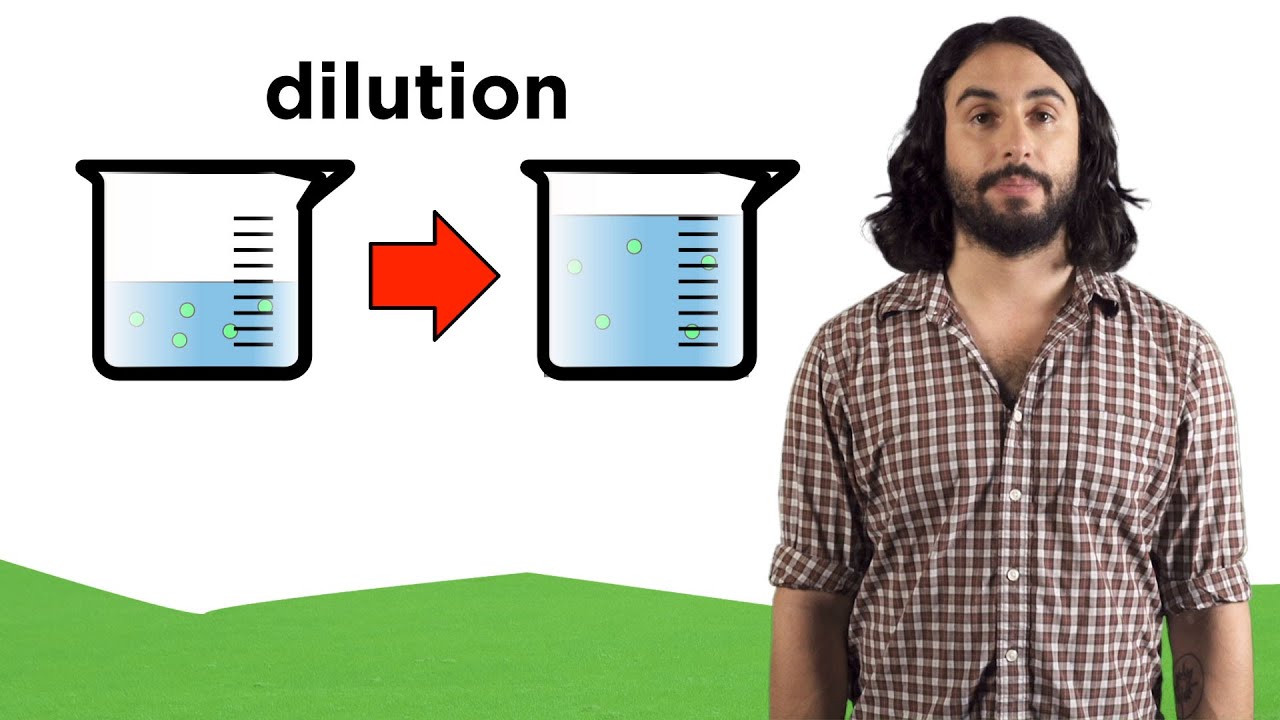
Показать описание
Now those pesky moles are swimming! But how much solute is there? Let's learn about how we measure concentrations of solutions, and how to perform dilution calculations.
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Molarity and Dilution
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
4.4 Molarity and Dilutions | General Chemistry
Molarity Made Easy: How to Calculate Molarity and Make Solutions
Molarity and Dilution Calculations
Molarity, Solution Stoichiometry and Dilution Problem
Molarity Practice Problems
Molarity and Dilution
Class - 6 | Molarity and its different numericals
Dilution Problems - Chemistry Tutorial
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
Calculating Molarity by Dilution
Molarity and Dilution Calculations
Molarity and Dilution.
Molarity and Dilution
Molarity: A Deeper Understanding
Molarity of dilution | Molarity | #shorts #iitjee #jee #neet #chemistry
How to Dilute a Solution
Molarity and Dilution: A Guide for Beginners
What Are Dilutions | Chemical Calculations | Chemistry | FuseSchool
Concentration and Molarity: The Key to Chemical Solutions
molarity and dilution
Concentration and Molarity explained: what is it, how is it used + practice problems
Molarity and Dilution Notes
Комментарии
 0:02:30
0:02:30
 0:21:55
0:21:55
 0:16:12
0:16:12
 0:08:46
0:08:46
 0:07:28
0:07:28
 0:10:25
0:10:25
 0:21:27
0:21:27
 0:48:48
0:48:48
 1:02:49
1:02:49
 0:06:14
0:06:14
 0:31:25
0:31:25
 0:08:40
0:08:40
 0:09:12
0:09:12
 0:03:00
0:03:00
 0:06:40
0:06:40
 0:11:51
0:11:51
 0:00:19
0:00:19
 0:03:36
0:03:36
 0:05:51
0:05:51
 0:03:51
0:03:51
 0:10:21
0:10:21
 0:07:07
0:07:07
 0:05:41
0:05:41
 0:23:52
0:23:52