filmov
tv
Trick to Calculate Molarity | Molarity practice problems

Показать описание
This lecture is about trick to calculate molarity in chemistry. I will teach you many numerical problems of molarity. After watching this lecture, you will be able to easily calculate molarity of any solution.
To learn more, watch this lecture till the end.
#molarity
#chemistry
#najamacademy
Join this channel to get access to perks:
To learn more, watch this lecture till the end.
#molarity
#chemistry
#najamacademy
Join this channel to get access to perks:
Trick to Calculate Molarity | Molarity practice problems
Molarity Made Easy: How to Calculate Molarity and Make Solutions
Super Easy Trick || How to Calculate Molarity in 2 Minutes || Solve Every Problems of Molarity
Molarity Practice Problems
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
How to Calculate Molarity With Tricks | How to Calculate Molarity | Molarity Calculation Tricks
Molarity - Chemistry Tutorial
molarity class 11 chemistry
Molarity Can Be Hard To Understand. Let's Visualize it.
Molarity and Molality formula tricks
How to calculate MOLARITY 😎 #chemistry #science #education #shorts
Calculating Molarity | Molar Concentration #chemistry #science #homework #shorts #short #education
How to find molarity in 10 seconds part 1
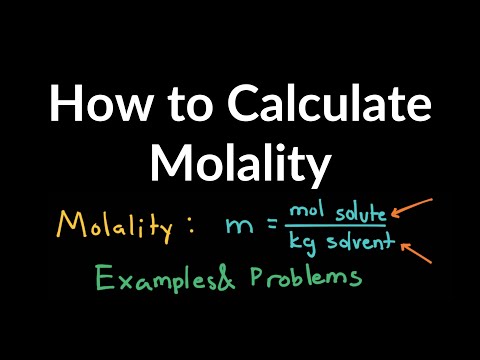
How to Calculate Molality of Solutions Examples, Practice Problems, Equation, Shortcut, Explanation
Easy Trick To Calculate Molarity 😃 | #shorts #chemistry #shortsfeed #studytips #moleconcept #tricks...
Calculating MOLES from MOLARITY 🥳💧#chemistry #science #education #youtubeshort #shorts
Molarity from Mass % and Density - Calculate Molarity from Mass Percent and Density
Difference between Molarity and Molality
Tricks to Solve Molarity based Questions Easily
Molarity, Molality, Normality Concentration Terms || Chapter-1 Solutions Class12 Chemistry #shorts
Direct way to calculate molarity from mass percent and density
Calculate the molarity of the solution
What is Molarity || Class 12th Chemistry || Alakh Pandey Sir || @Alakh Sir Highlights
How to calculate Molarity? | Molarity
Комментарии
 0:09:36
0:09:36
 0:08:46
0:08:46
 0:04:11
0:04:11
 0:21:27
0:21:27
 0:31:25
0:31:25
 0:15:36
0:15:36
 0:04:54
0:04:54
 0:00:50
0:00:50
 0:06:29
0:06:29
 0:00:40
0:00:40
 0:00:57
0:00:57
 0:00:58
0:00:58
 0:00:59
0:00:59
 0:05:25
0:05:25
 0:00:32
0:00:32
 0:00:38
0:00:38
 0:08:21
0:08:21
 0:04:07
0:04:07
 0:17:25
0:17:25
 0:00:58
0:00:58
 0:00:58
0:00:58
 0:00:58
0:00:58
 0:04:32
0:04:32
 0:01:00
0:01:00