filmov
tv
Is SO2 Polar or Nonpolar?

Показать описание
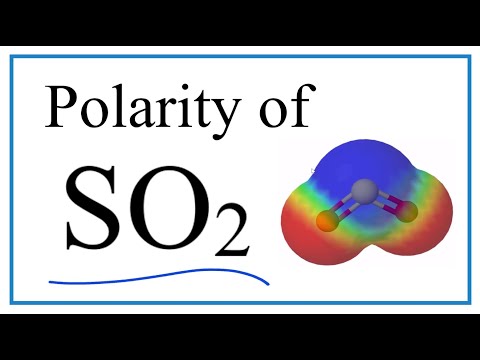
If you look at the Lewis structure for SO2 we can see that it is not a symmetrical molecule. While the left and right sides are the same there is a lone pair of electrons on the top of the SO2 molecule.
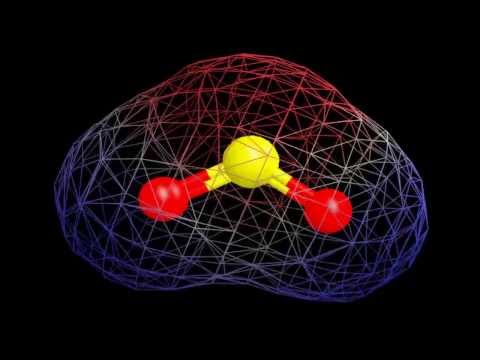
To determine if SO2 is polar we need to look at the molecular geometry or shape of the molecule. Polarity results from an unequal sharing of valence electrons. Because the SO2 molecule is not symmetrical there is a region of unequal sharing. The bent shape means that the top (where the lone pair of electron is) less electronegative. The Oxygen atoms at the bottom of the structure are then more negative. Therefore, SO2 is a polar molecule.
To determine if SO2 is polar we need to look at the molecular geometry or shape of the molecule. Polarity results from an unequal sharing of valence electrons. Because the SO2 molecule is not symmetrical there is a region of unequal sharing. The bent shape means that the top (where the lone pair of electron is) less electronegative. The Oxygen atoms at the bottom of the structure are then more negative. Therefore, SO2 is a polar molecule.
Is SO2 Polar or Non-Polar?
Is SO2 Polar or Nonpolar? (Sulfur Dioxide)
Is SO2 Polar or Nonpolar?
Is SO2 Polar or Nonpolar? | Molecular polarity for SO2 - Dr K
Is Sulfur Dioxide (SO2) Polar or Non-Polar? Lewis Structure
dipole moment: SO2 and SO3 | Polar | Non-Polar
Polar and Nonpolar Molecules
Is Sulfur Trioxide (SO3) Polar or Non-Polar? Lewis Structure (The Difference Between SO2 and SO3)
Polar And NonPolar Molecules | Chemistry
SO2 Lewis Structure,Valence Electrons,Formal Charge,Shape, Hybridization,Polar or Nonpolar,Octet Ru
Is CO2 a Polar or Non-Polar Molecule?
Is H2 Polar or Non-polar?
SO2, SO3 and Dipole Moment
Is CS2 polar or nonpolar?
Is CS2 polar or nonpolar?
Is SiCl4 Polar or Non-Polar?
Trick to identify polar and nonpolar molecules | How to identify polar and nonpolar molecules
Is NO2+ Polar or Nonpolar?
Molecular Dipole Moment Example 1 (CO, CO2, and SO2)
Is SO3 Polar or Nonpolar (Sulfur Trioxide)
#shorts# so2 &SO3 polar/ non polar molecules use Dipolemoment concept@ Veena Dixit Chemistry IIT...
Is SF2 Polar or Nonpolar? (Sulfur Difluoride)
sf4 polar or nonpolar
Is Nitric oxide (NO) Polar or Non-Polar?
Комментарии
 0:02:18
0:02:18
 0:01:38
0:01:38
 0:01:14
0:01:14
 0:01:29
0:01:29
 0:05:20
0:05:20
 0:00:53
0:00:53
 0:13:49
0:13:49
 0:07:12
0:07:12
 0:08:47
0:08:47
 0:16:14
0:16:14
 0:01:59
0:01:59
 0:01:01
0:01:01
 0:04:20
0:04:20
 0:01:03
0:01:03
 0:03:49
0:03:49
 0:01:51
0:01:51
 0:06:50
0:06:50
 0:01:56
0:01:56
 0:11:20
0:11:20
 0:01:59
0:01:59
 0:01:01
0:01:01
 0:01:24
0:01:24
 0:02:28
0:02:28
 0:01:11
0:01:11