filmov
tv
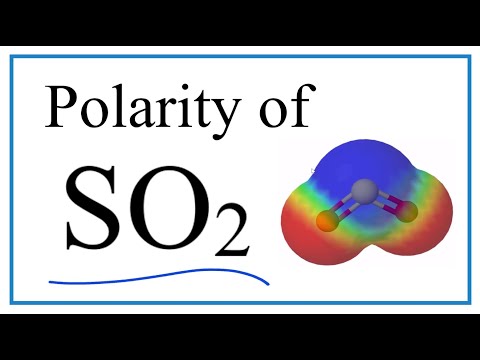
Is SO2 Polar or Non-Polar?

Показать описание
Learn to determine if SO2 (Sulfur dioxide) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).
We start with the Lewis Structure and look and the polarity of the individual bonds in SO2 based on the electronegativity difference between atoms. Then we will use VSEPR to determine the shape of the molecule and look at how the shape of the molecule allows us to determine if the entire molecule is polar or nonpolar.
More Learning Resources
We start with the Lewis Structure and look and the polarity of the individual bonds in SO2 based on the electronegativity difference between atoms. Then we will use VSEPR to determine the shape of the molecule and look at how the shape of the molecule allows us to determine if the entire molecule is polar or nonpolar.
More Learning Resources
Is SO2 Polar or Non-Polar?
Is SO2 Polar or Nonpolar? (Sulfur Dioxide)
Is SO2 Polar or Nonpolar? | Molecular polarity for SO2 - Dr K
Is Sulfur Dioxide (SO2) Polar or Non-Polar? Lewis Structure
Is SO2 Polar or Nonpolar?
dipole moment: SO2 and SO3 | Polar | Non-Polar
Polar and Nonpolar Molecules
Why SO2 is polar but SO3 is non-polar? -SO2 vs. SO3: Polar vs. Non-Polar Molecules Explained!
Polar And NonPolar Molecules | Chemistry
Is SiCl4 Polar or Non-Polar?
Trick to identify polar and nonpolar molecules | How to identify polar and nonpolar molecules
Is Sulfur Trioxide (SO3) Polar or Non-Polar? Lewis Structure (The Difference Between SO2 and SO3)
Why CO2 is non-polar and SO2 is Polar ? (XI Chemistry) Lecture 2
Is CS2 polar or nonpolar?
Is H2 Polar or Non-polar?
Is SBr2 Polar or Non-Polar (Sulfur dibromide)
SO2 Lewis Structure,Valence Electrons,Formal Charge,Shape, Hybridization,Polar or Nonpolar,Octet Ru
Is N2 Polar or Non-polar? (Nitrogen Gas)
Is CO2 a Polar or Non-Polar Molecule?
Is O2 Polar or Nonpolar (Oxygen)
Is this molecule polar or non polar 🤷♂️ ? @swarnchemistryclasses9734
Is Hydrogen Sulfide (H2S) Polar or Non-Polar? Lewis Structure
#shorts# so2 &SO3 polar/ non polar molecules use Dipolemoment concept@ Veena Dixit Chemistry IIT...
Is Nitric oxide (NO) Polar or Non-Polar?
Комментарии
 0:02:18
0:02:18
 0:01:38
0:01:38
 0:01:29
0:01:29
 0:05:20
0:05:20
 0:01:14
0:01:14
 0:00:53
0:00:53
 0:13:49
0:13:49
 0:03:59
0:03:59
 0:08:47
0:08:47
 0:01:51
0:01:51
 0:06:50
0:06:50
 0:07:12
0:07:12
 0:02:40
0:02:40
 0:01:03
0:01:03
 0:01:01
0:01:01
 0:01:38
0:01:38
 0:16:14
0:16:14
 0:01:50
0:01:50
 0:01:59
0:01:59
 0:01:32
0:01:32
 0:00:13
0:00:13
 0:04:14
0:04:14
 0:01:01
0:01:01
 0:01:11
0:01:11