filmov
tv
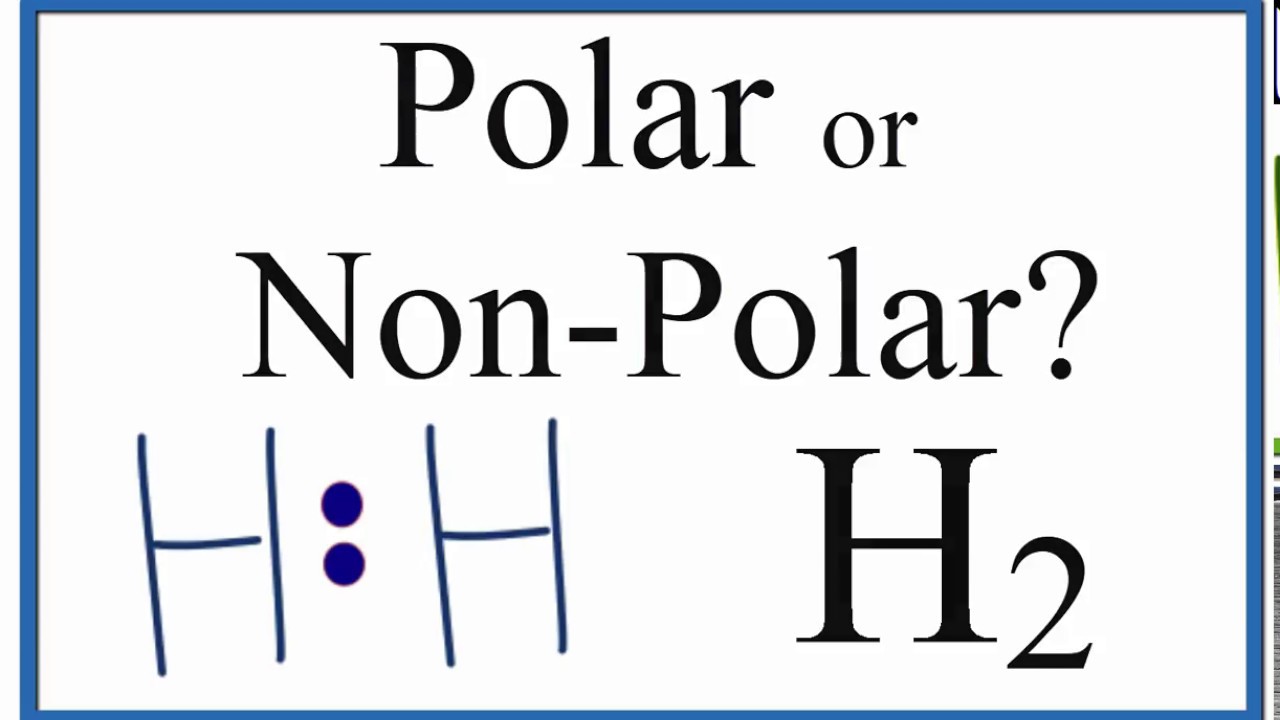
Is H2 Polar or Non-polar?
Показать описание
Learn to determine if H2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).
We start with the Lewis Structure and look and the polarity of the individual bonds in Hydrogen gas based on the electronegativity difference between atoms. Then we will use VSEPR to determine the shape of the molecule and look at how the shape of the molecule allows us to determine if the entire molecule is polar or nonpolar.
If you look at the Lewis Structure for H2 it appears to be a symmetrical molecule. However, to determine if H2 is polar we consider the molecular geometry. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons. For Hydrogen gas the molecule is symmetrical and therefore it is a nonpolar molecule.
While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like H2 these bonds are evenly distributed and cancel out. There is no net dipole and the H2 is non-polar.
More Learning Resources
We start with the Lewis Structure and look and the polarity of the individual bonds in Hydrogen gas based on the electronegativity difference between atoms. Then we will use VSEPR to determine the shape of the molecule and look at how the shape of the molecule allows us to determine if the entire molecule is polar or nonpolar.
If you look at the Lewis Structure for H2 it appears to be a symmetrical molecule. However, to determine if H2 is polar we consider the molecular geometry. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons. For Hydrogen gas the molecule is symmetrical and therefore it is a nonpolar molecule.
While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like H2 these bonds are evenly distributed and cancel out. There is no net dipole and the H2 is non-polar.
More Learning Resources
Is H2 Polar or Non-polar?
H2 Polar or NonPolar: Polarity Explained
Polar And NonPolar Molecules | Chemistry
Polar and Nonpolar Covalent Bonds
Is H2O polar or nonpolar?
4.2 Polar and non-polar molecules (SL)
S2.2.6 Polar and non-polar molecules
A Level H2 Chemistry , 2018, P1,Q3 - Determining Polar and Non Polar molecules via dipole moments
Trick to identify polar and nonpolar molecules | How to identify polar and nonpolar molecules
Chemistry: What is a Covalent Bond? - Polar & Nonpolar - Intramolecular Forces
Polar and Nonpolar Molecules
What is the Difference Between Polar and Non - Polar Substances | Chemistry Concepts
Is C2H2 Polar or Non-polar? (Ethyne or Acetylene)
Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice Problems
Nonpolar Covalent Bonds Explained
Is H2 (Hydrogen Gas) Ionic or Covalent/Molecular?
H2 (hydrogen gas) Lewis dot structure and polarity
Polar vs Non-Polar Covalent Bonds: The Ultimate Showdown
How to Identify the Intermolecular Force a Compound Has: London Dispersion, Dipole Dipole, H-Bonding
Is O2 Polar or Non-polar? (Oxygen Gas)
How to calculate electronegativity (EN)
S2.2.5 Polar and non-polar covalent bonds
Non polar covalent bond Animation#chemistry #animation #chemistryeducation #shoryoutube #youtube
How to Determine Type of Intermolecular Forces and Polarity of Molecules - H2ChemHacks
Комментарии
 0:01:01
0:01:01
 0:00:56
0:00:56
 0:08:47
0:08:47
 0:11:20
0:11:20
 0:01:11
0:01:11
 0:04:41
0:04:41
 0:07:02
0:07:02
 0:03:23
0:03:23
 0:06:50
0:06:50
 0:06:30
0:06:30
 0:12:42
0:12:42
 0:02:19
0:02:19
 0:01:18
0:01:18
 0:11:21
0:11:21
 0:00:54
0:00:54
 0:01:19
0:01:19
 0:01:20
0:01:20
 0:00:55
0:00:55
 0:05:37
0:05:37
 0:00:47
0:00:47
 0:01:50
0:01:50
 0:07:01
0:07:01
 0:00:30
0:00:30
 0:06:34
0:06:34