filmov
tv
Is CS2 polar or nonpolar?

Показать описание
CS2 is a linear molecule and the Sulfur (S) atoms on each end are symmetrical. Polarity results from an unequal sharing of valence electrons. Because of this symmetry there is no region of unequal sharing and CS2 is a nonpolar molecule.
Is CS2 polar or nonpolar?
Is CS2 polar or nonpolar?
Is CS2 polar or nonpolar? (Carbon Disulfide)
Is CS2 polar or nonpolar? (Carbon Disulfide)
Is Nitric oxide (NO) Polar or Non-Polar?
Is PCL3 Polar or Nonpolar? (Phosphorus Trichloride)
Is SiF4 Polar or Nonpolar? (Silicon tetrafluoride)
Polar, Non-Polar, and Ionic Compounds: Explanation, Examples, and Practice
Is SiCl2F2 polar or nonpolar? Check Polarity
Is O3 Polar or Nonpolar?
Is SF2 Polar or Nonpolar?
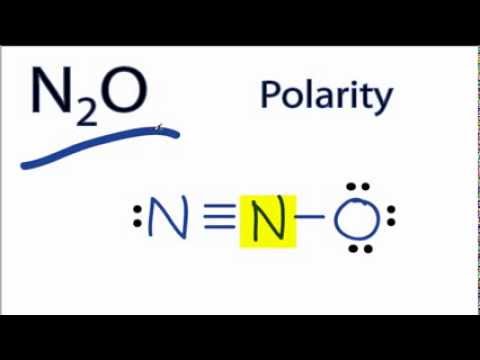
Is N2O Polar or Nonpolar?
Is ClF Polar or Nonpolar (Chlorine Monoluoride)
Is HNO2 Polar or Nonpolar?
Is BF3 Polar or Non-polar? (Boron Trifluoride)
Is SCl2 a polar or nonpolar molecule?
Is SiCl4 Polar or Non-Polar?
Is SO2 Polar or Nonpolar?
Is H2 Polar or Non-polar?
Is SiF4 polar or non-polar?
Is SBr2 Polar or Non-Polar (Sulfur Dibromide)
Is Sulfur Dioxide (SO2) Polar or Non-Polar? Lewis Structure
Is O3 Polar or Non-polar? (Ozone)
Is this molecule polar or non polar 🤷♂️ ? @swarnchemistryclasses9734
Комментарии
 0:01:03
0:01:03
 0:03:49
0:03:49
 0:01:14
0:01:14
 0:01:14
0:01:14
 0:01:11
0:01:11
 0:01:28
0:01:28
 0:01:59
0:01:59
 0:09:37
0:09:37
 0:01:32
0:01:32
 0:00:55
0:00:55
 0:01:20
0:01:20
 0:00:47
0:00:47
 0:01:50
0:01:50
 0:01:36
0:01:36
 0:01:29
0:01:29
 0:06:37
0:06:37
 0:01:51
0:01:51
 0:01:14
0:01:14
 0:01:01
0:01:01
 0:02:41
0:02:41
 0:01:50
0:01:50
 0:05:20
0:05:20
 0:01:22
0:01:22
 0:00:13
0:00:13