filmov
tv
Writing Ionic Compounds Formulas | Fast & Easy Way | Practice Examples | Chemistry

Показать описание
Science 9 - Unit B: Matter & Chemical Changes
4 simple steps to write formulas for ionic compounds with practice examples.
Step 1: Write down the metal ion first with its charge.
Step 2: Write down the non-metal ion second with its charge.
Step 3: Keep adding either metal ions or non-metal ions until the positive and negative charges balances out, they must add up to zero.
Step 4: Count the number of ions of each element. Put the number as a subscript to the right of the ion symbol and remove the ion charge.
*Note: the number “1” is invisible
Answer for practice questions:
SnF₂
AlPO₄
CaS
LiBr
BeF₂
4 simple steps to write formulas for ionic compounds with practice examples.
Step 1: Write down the metal ion first with its charge.
Step 2: Write down the non-metal ion second with its charge.
Step 3: Keep adding either metal ions or non-metal ions until the positive and negative charges balances out, they must add up to zero.
Step 4: Count the number of ions of each element. Put the number as a subscript to the right of the ion symbol and remove the ion charge.
*Note: the number “1” is invisible
Answer for practice questions:
SnF₂
AlPO₄
CaS
LiBr
BeF₂
Writing Chemical Formulas For Ionic Compounds
Writing Ionic Formulas: Introduction
How to Write Formulas for Simple Ionic Compounds | Breslyn.org
Writing Ionic Formulas with Transition Metals
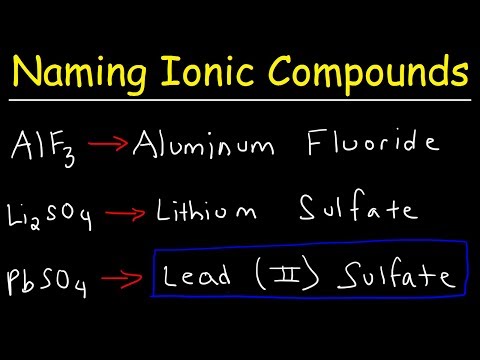
How To Name Ionic Compounds With Transition Metals
How To Write Ionic Formulas With Polyatomic Ions
The Criss-Cross Method for Writing Chemical Formulas
Naming Ionic and Molecular Compounds | How to Pass Chemistry
Naming Ionic Compounds
Writing Formulas for Ionic and Molecular Compounds in 15 Minutes
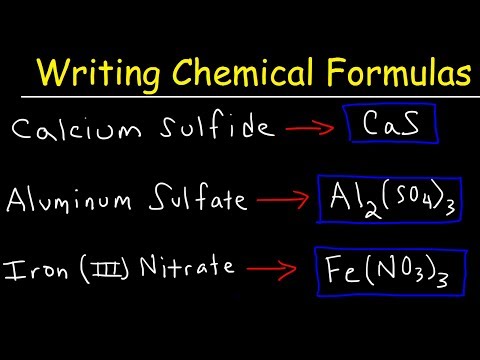
Writing Formulas for Ionic Compounds
Formulae Of Ionic Compounds & Their Names - Part 1 | Properties of Matter | Chemistry | FuseScho...
Writing Ionic Formulas: Practice Problems
Criss-Cross Method for Writing Formulas for Ionic Compounds
Writing Formulas for Ionic Compounds
Naming and writing ionic formulas
GCSE Chemistry - What is an Ionic Compound? Ionic Compounds Explained #15
Writing Ionic Formulas, Naming Ionic Compounds & Covalent Compounds
Writing Ionic Compounds Formulas | Fast & Easy Way | Practice Examples | Chemistry
Writing Ionic Compound Names and Formulas: Tips and Examples
Writing Formulas with Polyatomic Ions
Ionic Compounds: Writing Chemical Formulas
Writing the Formulas of Ionic Compounds Simplified
Chemistry 5.3 Formula Writing: Ionic Compounds
Комментарии
 0:10:22
0:10:22
 0:11:44
0:11:44
 0:04:39
0:04:39
 0:07:21
0:07:21
 0:13:33
0:13:33
 0:10:41
0:10:41
 0:05:30
0:05:30
 0:10:32
0:10:32
 0:05:44
0:05:44
 0:14:26
0:14:26
 0:16:52
0:16:52
 0:03:23
0:03:23
 0:03:42
0:03:42
 0:05:05
0:05:05
 0:04:48
0:04:48
 0:04:32
0:04:32
 0:06:08
0:06:08
 0:17:56
0:17:56
 0:03:37
0:03:37
 0:04:55
0:04:55
 0:11:21
0:11:21
 0:08:43
0:08:43
 0:11:44
0:11:44
 0:08:12
0:08:12