filmov
tv
Writing Ionic Compound Names and Formulas: Tips and Examples

Показать описание
In this video we look at a brief overview of naming and writing formulas for simple ionic compounds.
Naming and Formula Writing Playlist:
Join this channel to get access to perks:
1. Identify the Cation (Metal) and Anion (Nonmetal):
In a binary ionic compound, the cation is usually a metal, and the anion is a nonmetal.
2. Determine Ionic Charges:
Find the charges of the cation and anion based on their positions in the periodic table or given information. Metals typically form cations with positive charges, and nonmetals form anions with negative charges.
3. Balance Charges:
The charges of the cation and anion should balance to form a neutral compound. This means the magnitude of the positive charge on the cation should equal the magnitude of the negative charge on the anion.
4. Write the Compound Formula:
Write the symbol of the cation followed by the symbol of the anion. Use subscripts to indicate the ratio of ions needed to balance the charges. The subscripts should be the smallest whole numbers that result in a neutral compound.
5. Name the Compound:
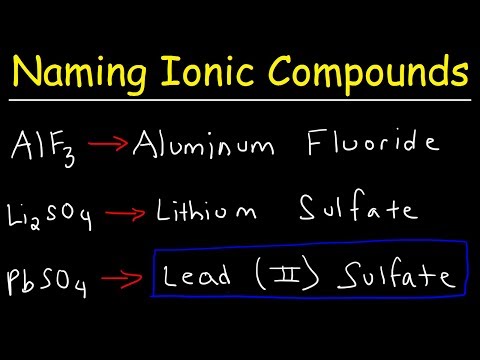
For the cation (metal), simply use its name. For the anion (nonmetal), change the ending to "-ide."
6. Examples:
Example 1: Sodium Chloride
Cation: Sodium (Na+)
Anion: Chlorine (Cl-)
Balanced Charges: Na+ + Cl- = NaCl
Compound Name: Sodium chloride
Example 2: Magnesium Oxide
Cation: Magnesium (Mg2+)
Anion: Oxygen (O2-)
Balanced Charges: Mg2+ + O2- = MgO
Compound Name: Magnesium oxide
Example 3: Aluminum Sulfide
Cation: Aluminum (Al3+)
Anion: Sulfur (S2-)
Balanced Charges: 2 Al3+ + 3 S2- = Al2S3
Compound Name: Aluminum sulfide
Example 4: Potassium Nitride
Cation: Potassium (K+)
Anion: Nitrogen (N3-)
Balanced Charges: 3 K+ + N3- = K3N
Compound Name: Potassium nitride
Remember that the overall charge of the compound should be neutral, so you may need to adjust the subscripts to achieve this balance. Always check that the charges cancel out and the sum of the positive charges equals the sum of the negative charges.
Understanding the ionic charges, the distinction between metals and nonmetals, and the concept of charge balancing are crucial for correctly naming and writing formulas for simple binary ionic compounds.
Naming and Formula Writing Playlist:
Join this channel to get access to perks:
1. Identify the Cation (Metal) and Anion (Nonmetal):
In a binary ionic compound, the cation is usually a metal, and the anion is a nonmetal.
2. Determine Ionic Charges:
Find the charges of the cation and anion based on their positions in the periodic table or given information. Metals typically form cations with positive charges, and nonmetals form anions with negative charges.
3. Balance Charges:
The charges of the cation and anion should balance to form a neutral compound. This means the magnitude of the positive charge on the cation should equal the magnitude of the negative charge on the anion.
4. Write the Compound Formula:
Write the symbol of the cation followed by the symbol of the anion. Use subscripts to indicate the ratio of ions needed to balance the charges. The subscripts should be the smallest whole numbers that result in a neutral compound.
5. Name the Compound:
For the cation (metal), simply use its name. For the anion (nonmetal), change the ending to "-ide."
6. Examples:
Example 1: Sodium Chloride
Cation: Sodium (Na+)
Anion: Chlorine (Cl-)
Balanced Charges: Na+ + Cl- = NaCl
Compound Name: Sodium chloride
Example 2: Magnesium Oxide
Cation: Magnesium (Mg2+)
Anion: Oxygen (O2-)
Balanced Charges: Mg2+ + O2- = MgO
Compound Name: Magnesium oxide
Example 3: Aluminum Sulfide
Cation: Aluminum (Al3+)
Anion: Sulfur (S2-)
Balanced Charges: 2 Al3+ + 3 S2- = Al2S3
Compound Name: Aluminum sulfide
Example 4: Potassium Nitride
Cation: Potassium (K+)
Anion: Nitrogen (N3-)
Balanced Charges: 3 K+ + N3- = K3N
Compound Name: Potassium nitride
Remember that the overall charge of the compound should be neutral, so you may need to adjust the subscripts to achieve this balance. Always check that the charges cancel out and the sum of the positive charges equals the sum of the negative charges.
Understanding the ionic charges, the distinction between metals and nonmetals, and the concept of charge balancing are crucial for correctly naming and writing formulas for simple binary ionic compounds.
Комментарии
 0:13:33
0:13:33
 0:05:44
0:05:44
 0:10:22
0:10:22
 0:10:32
0:10:32
 0:11:44
0:11:44
 0:10:10
0:10:10
 0:11:25
0:11:25
 0:03:57
0:03:57
 0:29:47
0:29:47
 0:04:55
0:04:55
 0:04:27
0:04:27
 0:10:43
0:10:43
 0:17:56
0:17:56
 0:07:21
0:07:21
 0:03:23
0:03:23
 0:16:04
0:16:04
 0:07:14
0:07:14
 0:06:05
0:06:05
 0:01:30
0:01:30
 0:11:58
0:11:58
 0:04:32
0:04:32
 0:09:38
0:09:38
 0:06:52
0:06:52
 0:07:45
0:07:45