filmov
tv
Naming ions and ionic compounds | Atoms, compounds, and ions | Chemistry | Khan Academy

Показать описание
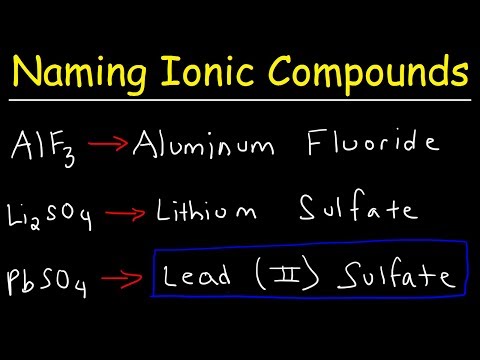
Ionic compounds are neutral compounds made up of positively charged ions called cations and negatively charged ions called anions. For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion. For example, KCl, an ionic compound that contains K⁺ and Cl⁻ ions, is named potassium chloride.
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help! Donate or volunteer today!
Комментарии
 0:05:44
0:05:44
 0:13:33
0:13:33
 0:03:57
0:03:57
 0:10:32
0:10:32
 0:10:22
0:10:22
 0:10:10
0:10:10
 0:11:25
0:11:25
 0:10:43
0:10:43
 0:04:02
0:04:02
 0:11:44
0:11:44
 0:16:04
0:16:04
 0:02:15
0:02:15
 0:01:30
0:01:30
 0:03:23
0:03:23
 0:04:27
0:04:27
 0:06:08
0:06:08
 0:29:45
0:29:45
 0:00:53
0:00:53
 0:11:58
0:11:58
 0:08:13
0:08:13
 0:11:56
0:11:56
 0:02:22
0:02:22
 0:06:05
0:06:05
 0:05:08
0:05:08