filmov
tv
Naming Ionic Compounds, a tutorial | Crash Chemistry Academy

Показать описание
This video demonstrates how to name all varieties of ionic compounds you will encounter in your class: differences in naming positive ions with one possible charge versus more than one possible charge, differences in naming binary versus ternary ionic compounds (= compounds with polyatomic ions).
Useful links for ion naming from the video:
—More on Ionic Compounds | Wiki —
"In chemistry, an ionic compound is a chemical compound comprising ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network, usually in a crystalline structure.
Ionic compounds containing hydrogen ions (H+) are classified as acids, and those containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases. Ionic compounds without these ions are also known as salts and can be formed by acid–base reactions. Ionic compounds can also be produced from their constituent ions by evaporation of their solvent, precipitation, freezing, a solid-state reaction, or the electron transfer reaction of reactive metals with reactive non-metals, such as halogen gases.
Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids they are almost always electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
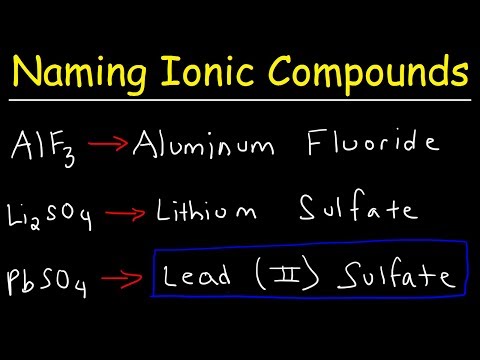
According to the nomenclature recommended by IUPAC, ionic compounds are named according to their composition, not their structure.[94] In the most simple case of a binary ionic compound with no possible ambiguity about the charges and thus the stoichiometry, the common name is written using two words.[95] The name of the cation (the unmodified element name for monatomic cations) comes first, followed by the name of the anion.[96][97] For example, MgCl2 is named magnesium chloride, and Na2SO4 is named sodium sulfate (SO2−
4, sulfate, is an example of a polyatomic ion). To obtain the empirical formula from these names, the stoichiometry can be deduced from the charges on the ions, and the requirement of overall charge neutrality.
If there are multiple cations and/or anions, multiplicative prefixes (di-, tri-, tetra-, ...) are often required to indicate the relative compositions,[98] and cations then anions are listed in alphabetical order.[99] For example, KMgCl3 is named magnesium potassium trichloride (note that in both the empirical formula and the written name, the cations appear in alphabetical order, but the order varies between them because the symbol for potassium is K).[100] When one of the ions already has a multiplicative prefix in its name, the alternate multiplicative prefixes (bis-, tris-, tetrakis-, ...) are used.[101] For example, Ba(BrF4)2 is named barium bis(tetrafluoridobromate).[102] "
Wikipedia contributors. "Ionic compound." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 4 Apr. 2016. Web. 13 Jul. 2016.
Useful links for ion naming from the video:
—More on Ionic Compounds | Wiki —
"In chemistry, an ionic compound is a chemical compound comprising ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network, usually in a crystalline structure.
Ionic compounds containing hydrogen ions (H+) are classified as acids, and those containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases. Ionic compounds without these ions are also known as salts and can be formed by acid–base reactions. Ionic compounds can also be produced from their constituent ions by evaporation of their solvent, precipitation, freezing, a solid-state reaction, or the electron transfer reaction of reactive metals with reactive non-metals, such as halogen gases.
Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids they are almost always electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
According to the nomenclature recommended by IUPAC, ionic compounds are named according to their composition, not their structure.[94] In the most simple case of a binary ionic compound with no possible ambiguity about the charges and thus the stoichiometry, the common name is written using two words.[95] The name of the cation (the unmodified element name for monatomic cations) comes first, followed by the name of the anion.[96][97] For example, MgCl2 is named magnesium chloride, and Na2SO4 is named sodium sulfate (SO2−
4, sulfate, is an example of a polyatomic ion). To obtain the empirical formula from these names, the stoichiometry can be deduced from the charges on the ions, and the requirement of overall charge neutrality.
If there are multiple cations and/or anions, multiplicative prefixes (di-, tri-, tetra-, ...) are often required to indicate the relative compositions,[98] and cations then anions are listed in alphabetical order.[99] For example, KMgCl3 is named magnesium potassium trichloride (note that in both the empirical formula and the written name, the cations appear in alphabetical order, but the order varies between them because the symbol for potassium is K).[100] When one of the ions already has a multiplicative prefix in its name, the alternate multiplicative prefixes (bis-, tris-, tetrakis-, ...) are used.[101] For example, Ba(BrF4)2 is named barium bis(tetrafluoridobromate).[102] "
Wikipedia contributors. "Ionic compound." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 4 Apr. 2016. Web. 13 Jul. 2016.
Комментарии
 0:05:44
0:05:44
 0:13:33
0:13:33
 0:06:52
0:06:52
 0:10:32
0:10:32
 0:10:22
0:10:22
 0:11:25
0:11:25
 0:00:53
0:00:53
 0:03:57
0:03:57
 0:10:10
0:10:10
 0:04:27
0:04:27
 0:15:31
0:15:31
 0:05:26
0:05:26
 0:01:14
0:01:14
 0:05:08
0:05:08
 0:01:30
0:01:30
 0:07:45
0:07:45
 0:03:01
0:03:01
 0:03:31
0:03:31
 0:07:36
0:07:36
 0:03:30
0:03:30
 0:02:22
0:02:22
 0:06:39
0:06:39
 0:11:44
0:11:44
 0:31:24
0:31:24