filmov
tv
Electron Configuration

Показать описание
Need help preparing for the General Chemistry section of the MCAT? MedSchoolCoach expert, Ken Tao, will teach everything you need to know about Electron Configuration for Electronic Structure. Watch this video to get all the MCAT study tips you need to do well on this section of the exam!
Electron configuration is a topic that shows up pretty frequently on the MCAT and as long as we are familiar with the basic steps of writing out electron configuration, they can be easy and quick points. This requires us to understand both the rules for writing out the electron configuration, as well as a few notable exceptions.
Shells and Subshells
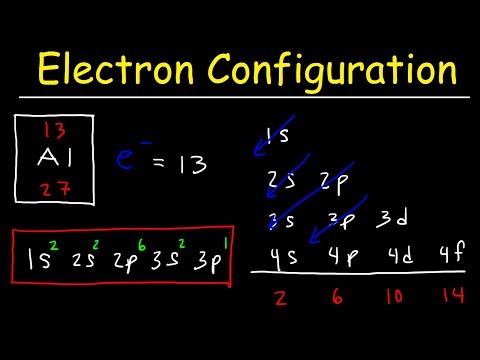
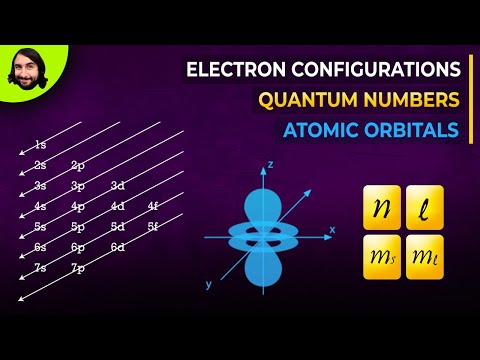
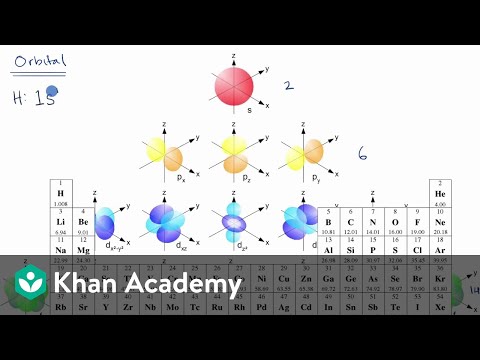
Electron configuration is a quick and easily identifiable way of describing which electron shells and subshells are filled in any given atom or ion, without going through the time and hassle of writing out the quantum configuration of every electron in every shell and subshell. Recall from our previous videos that electrons always occupy a quantized electron shell, or energy level, given by a principle quantum number n that increases with increasing periods, with the n = 1 shell corresponding to the first period, the n = 2 shell corresponding to the second period, and so on. Each shell can be further broken down into subshells, with the four primary subshells being the s-subshell (groups 1 and 2 of the periodic table, azimuthal quantum number 0), the p-subshell (groups 13-18, azimuthal quantum number 1), the d-subshell (primarily transition metals, azimuthal quantum number 2) and the f-subshell (lanthanides and actinides, azimuthal quantum number), each of which will have a different, discrete energy value.
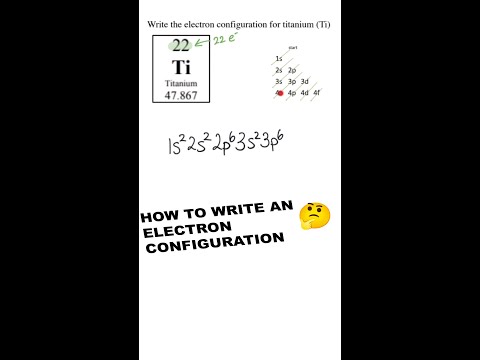
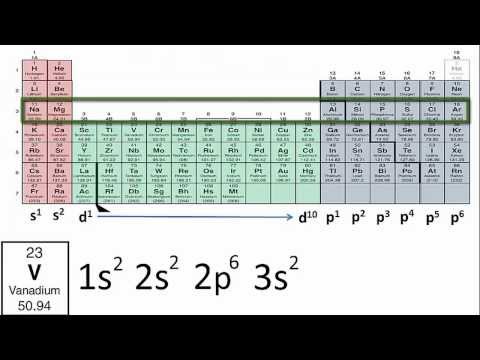
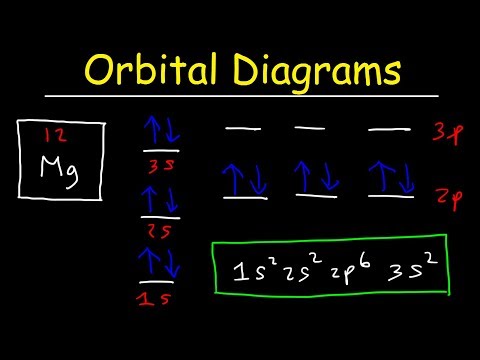
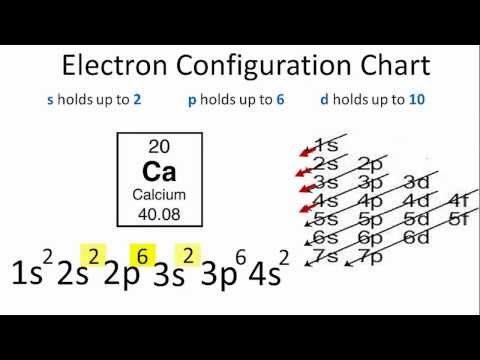
Each electron shell has a progressively higher energy level, but it turns out that we can also compare the energy levels of subshells to each other. The first electron shell only has one subshell, the s-subshell, which we can further specify as the 1s subshell, or the s-subshell within the 1 shell. This would be our lowest energy subshell possible. Moving up, we will then progress to the 2 shell, which consists of both a 2s subshell and a 2p subshell. The 2p subshell is more complex and higher energy than the 2s subshell, and so will be listed after. After this will come the 3 shell, consisting of the 3s subshell, the 3p subshell, and the 3d subshell. However, this is where we hit our first exception. Due to the increasing complexity of each subshell, and the decreasing gap in energy between each successive shell, it works out that the 4s subshell is actually a lower energy than the 3d subshell, even though we would usually classify the 4 shell as being of a higher energy than the 3 shell. Therefore, if we were to rank the first eight subshells from lowest to highest energy, it would progress 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p. Note that this pattern holds true for every d-subshell, with the numbering of each progressive d-subshell one lower than the period the atom or ion appears in.
Noble Gas Configuration
This notation can become a bit cumbersome for larger elements, and so we typically use a shorthand involving the electron configuration of noble gases. Consider as an example iron (atomic number 26). Elemental iron has an atomic configuration of 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d6. However, we can simply this by using the noble gas notation of the noble gas most immediately preceding it, in this case argon (atomic number 18). Argon has an atomic configuration of 1s2, 2s2, 2p6, 3s2, 3p6. This configuration can then be shown simply by putting argon’s elemental symbol (Ar), in brackets, denoting the aforementioned electronic configuration. Iron’s configuration could then be rewritten as [Ar] 4s2 3d6, showing the same configuration in much shorter notation.
MEDSCHOOLCOACH
To watch more MCAT video tutorials like this and have access to study scheduling, progress tracking, flashcard and question bank, download MCAT Prep by MedSchoolCoach
#medschoolcoach #MCATprep #MCATstudytools
Electron configuration is a topic that shows up pretty frequently on the MCAT and as long as we are familiar with the basic steps of writing out electron configuration, they can be easy and quick points. This requires us to understand both the rules for writing out the electron configuration, as well as a few notable exceptions.
Shells and Subshells
Electron configuration is a quick and easily identifiable way of describing which electron shells and subshells are filled in any given atom or ion, without going through the time and hassle of writing out the quantum configuration of every electron in every shell and subshell. Recall from our previous videos that electrons always occupy a quantized electron shell, or energy level, given by a principle quantum number n that increases with increasing periods, with the n = 1 shell corresponding to the first period, the n = 2 shell corresponding to the second period, and so on. Each shell can be further broken down into subshells, with the four primary subshells being the s-subshell (groups 1 and 2 of the periodic table, azimuthal quantum number 0), the p-subshell (groups 13-18, azimuthal quantum number 1), the d-subshell (primarily transition metals, azimuthal quantum number 2) and the f-subshell (lanthanides and actinides, azimuthal quantum number), each of which will have a different, discrete energy value.
Each electron shell has a progressively higher energy level, but it turns out that we can also compare the energy levels of subshells to each other. The first electron shell only has one subshell, the s-subshell, which we can further specify as the 1s subshell, or the s-subshell within the 1 shell. This would be our lowest energy subshell possible. Moving up, we will then progress to the 2 shell, which consists of both a 2s subshell and a 2p subshell. The 2p subshell is more complex and higher energy than the 2s subshell, and so will be listed after. After this will come the 3 shell, consisting of the 3s subshell, the 3p subshell, and the 3d subshell. However, this is where we hit our first exception. Due to the increasing complexity of each subshell, and the decreasing gap in energy between each successive shell, it works out that the 4s subshell is actually a lower energy than the 3d subshell, even though we would usually classify the 4 shell as being of a higher energy than the 3 shell. Therefore, if we were to rank the first eight subshells from lowest to highest energy, it would progress 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p. Note that this pattern holds true for every d-subshell, with the numbering of each progressive d-subshell one lower than the period the atom or ion appears in.
Noble Gas Configuration
This notation can become a bit cumbersome for larger elements, and so we typically use a shorthand involving the electron configuration of noble gases. Consider as an example iron (atomic number 26). Elemental iron has an atomic configuration of 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d6. However, we can simply this by using the noble gas notation of the noble gas most immediately preceding it, in this case argon (atomic number 18). Argon has an atomic configuration of 1s2, 2s2, 2p6, 3s2, 3p6. This configuration can then be shown simply by putting argon’s elemental symbol (Ar), in brackets, denoting the aforementioned electronic configuration. Iron’s configuration could then be rewritten as [Ar] 4s2 3d6, showing the same configuration in much shorter notation.
MEDSCHOOLCOACH
To watch more MCAT video tutorials like this and have access to study scheduling, progress tracking, flashcard and question bank, download MCAT Prep by MedSchoolCoach
#medschoolcoach #MCATprep #MCATstudytools
Комментарии
 0:10:19
0:10:19
 0:07:23
0:07:23
 0:10:17
0:10:17
 0:04:59
0:04:59
 0:08:42
0:08:42
 0:01:00
0:01:00
 0:20:21
0:20:21
 0:04:52
0:04:52
 0:00:16
0:00:16
 0:05:08
0:05:08
 0:05:24
0:05:24
 0:12:48
0:12:48
 0:03:25
0:03:25
 0:06:24
0:06:24
 0:12:12
0:12:12
 0:03:53
0:03:53
 0:17:24
0:17:24
 0:14:39
0:14:39
 0:12:13
0:12:13
 0:06:11
0:06:11
 0:12:57
0:12:57
 0:11:05
0:11:05
 0:30:45
0:30:45
 0:01:42
0:01:42